Hysteroscopic Sterilization
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Sterilization continues to be the most commonly used contraceptive method in the United States, with 11 million U.S. women relying on the method. Transvaginal approaches to sterilization involve gaining access to the fallopian tubes through the cervix. A device or occlusive material is then placed hysteroscopically or blindly block each tube. For many practicing obstetrician and gynecologists, tubal ligation was the gold standards -- both laparoscopic and postpartum -- have been performed safely and efficiently, and nearly all obstetricians and gynecologists are well versed in the procedure. Yet gynecologic surgeons have simultaneously sought to occlude the fallopian tubes transcervically to avoid the discomfort and complications associated with transabdominal approaches. Different techniques and devices have been tried, and varying degrees of success have been reported. From a practical perspective, to be considered and acceptable technique for permanent female contraception, transcervical sterilization needs to be judged against tubal ligation on four criteria: effectiveness; safety; discomfort and pain; and cost. With today's technology, transcervical sterilization can easily be performed both comfortably and cost-effectively in an office setting rather than an operating room, making sterilization a convenient and private choice for non-reversible birth control.
The purpose of this document is to review hysteroscopic sterilization. Past, current, and upcoming techniques are reviewed to determine how they measure up to tubal ligation. Although sterilization is intended to be permanent, expressions of regret and requests for reversal are not uncommon and are much likely to occur among women sterilized at young ages. The availability and use of contraception have contributed greatly to women's health. The emphasis will be on the safety and effectiveness of hysteroscopic sterilization as compared with the alternatives.
Transcervical Sterilization:
Numerous techniques and technologies have been applied to transcervical sterilization, with varying degrees of success. However, until recently, no method had become established due to high failure and complication rates. Some methods require visualization of the tubal ostia with hysteroscopy, while others do not. The Essure® permanent birth control device (Conceptus, Inc., Mountain View, California) is a relatively new method of minimally invasive transcervical sterilization that was approved by the U.S. Food and Drug administration in November 2002 (1). The majority of information on Essure® hysteroscopic sterilization is based on clinical trials. Newer methods are (2):
- Corrosive agents -- nitric acid, phenol, quinacrine, methylcyanoacrylate;
- Mechanical obstruction devices -- silicone, polyethylene, nylon, ceramic;
- Destruction using thermal energy -- electrocautery, YAG laser.
Advantages and Disadvantages of Hysteroscopic Sterilization:
Advantages of hysteroscopic sterilization over sterilization via laparoscopy or laparotomy are (3):
- No incision;
- Can be performed in an office setting so it is more cost- and time-effective;
- Minimal to no anesthetic requirements;
- Less post-operative pain;
- Can be performed in women with extensive pelvic adhesions;
- Can be performed in women with co-morbidities that preclude laparoscopy or laparotomy.
Disadvantages are (4):
- Need for contraception for 12 weeks post-procedure (until tubal occlusion is confirmed);
- Expense of device and imaging study to confirm tubal occlusion;
- Higher risk of unilateral tubal occlusion;
- Electrical conductivity of micro-insert limits the use of electrocautery during subsequent pelvic procedures (eg, endometrial ablation);
- Need for adequate vaginal surgical training to minimize potential complications.
Hysteroscopic Sterilization Device:
The only form of transcervical sterilization approved for use in the United States is the Essure® micro-insert device; it is also used in many other countries. The device is a metal and polymer micro-insert 4 cm long and 1 to 2 mm wide when deployed. It consists of an inner coil of stainless steel and polyethylene terephthalate (PET) fibers and outer coil of nickel-titanium (nitinol). It comes loaded in a single-use delivery system. The device is placed under hysteroscopic guidance in the proximal fallopian tube. The coil initially is in a tightly wound state and then is deployed to an expanded state that anchors the insert in the tube. After placement, the PET fibers stimulate benign tissue growth that surrounds and infiltrates the device over the course of several weeks, resulting in tubal occlusion (5). Twelve weeks after placement, a hysterosalpingogram (HSG) is performed to confirm tubal occlusion. Contraception must be used until satisfactory micro-insert location and bilateral tubal occlusion are confirmed.
Contraindications:
Contraindications to hysteroscopic sterilization include:
- Pregnancy or suspected pregnancy;
- Less than six weeks from a delivery or abortion (spontaneous or induced);
- Uncertainty about non-reversible sterilization;
- Active or recent pelvic infection;
- Uterine or tubal pathology that impedes access to one or both tubal ostia;
- Hypersensitivity to nickel confirmed by skin-test (6);
- Known allergy to contrast media (not able to undergo HSG to confirm tubal occlusion).
Patient Counseling and Pre-operative Preparation:
Patients considering hysteroscopic sterilization should be counseled regarding other methods of contraception and other approaches to female and male sterilization. In addition, it is important for a patient to understand that sterilization is not reversible and to discuss the possibility of future regret. In the current method of hysteroscopic sterilization, the micro-insert spans the entire length of the tube, and thus, it is not reversible. Women who desire pregnancy after the procedure will require in vitro fertilization (IVF). While there are few data, it appears that microinsert presence in the tubes does not diminish IVF success rates (7).
Pre-operative examination, cervical preparation and dilation, and surgical site infection prevention for hysteroscopic sterilization are similar as for other hysteroscopic procedures. Review informed consent regarding non-reversible contraception and surgical issues; conduct medical history and physical examination; and prescribe pre-procedure medications. Two or more weeks to procedure -- initiate or continue progestin-containing contraceptive or schedule procedure for follicular phase of menstrual cycle. One day prior to procedure -- oral non-steroidal anti-inflammatory drug (NSAID) (e.g. ibuprofen 600 mg every six hours; ketorolac on the procedure day is an alternative). Single dose of misoprostol 200 to 400 mcg per vaginum can be used at bedtime if needed to facilitate cervical dilation.
Procedure:
On the day of procedure -- rule out pregnancy. Administer ketorolac 30 mg intramuscularly or intravenously 15 minutes before the procedure (if patient has not taken oral NSAID within 24 hours). Check equipment - Essure® device; two individually wrapped devices, more should be available in case a device is damaged or does not deploy properly. Rigid hysteroscope must have a 12" or a 30" lens, fluid inflow channel, and instrument channel. Hysteroscopic grasper for use in an errantly deployed device needs to be retrieved from the uterine cavity. Electrolyte solution (e.g. normal saline or lactated Ringer's) for uterine distention, use fluid sparingly to avoid overload and endometrial edema that can obscure operative field; manual or automatic system to monitor inflow and fluid deficit. Transcervical sterilization is an outpatient procedure that can be comfortably performed in an office setting, under no or local anesthesia or paracervical block (8). Intravenous conscious sedation is a reversible option for patients who otherwise cannot tolerate the procedure.
Position and prepare patient for hysteroscopy. Perform routine hysteroscopic cervical preparation and dilation, insert the hysteroscope, briefly inspect the uterine cavity. Note whether both tubal ostia can be visualized and are accessible; also not any pathology. The device is for single-use and should not be opened until visualization of both ostia is confirmed. Throughout the procedure, the distention medium should be used as sparingly as possible. This will both reduce the risk of fluid overload and minimize endometrial tissue edema -- which can obscure the view of the ostia. Prevention of tissue edema, which can develop within several minutes, is an important way of ensuring success of the procedure. The micro-inserts are expensive and are easily damaged. Thus the surgeon or assistant before starting the procedure should check that the working channel is not blocked before inserting the device. This can be done by passing another instrument through the working channel. Insert (or have an assistant insert) the hysteroscopic sterilization device introducer sheath into the working channel of the hysteroscope. Position the hysteroscope in front of the one of the tubal ostia while the assistant threads the first device delivery system into the introducer sheath. With the delivery system threaded, take hold of the device handle and guide the tip of the micro-insert through the tubal ostium into the fallopian tube.
The maneuvers to correctly position and deploy the device must be performed precisely. While learning the procedure, it may be helpful to review the following steps before each patient (6):
- Thread the device into the tube until the black marker is positioned at the tubal ostium;
- Rotate the thumbwheel towards yourself until it can no longer rotate (this will expose the wound down micro-insert);
- Align the gold marker band just outside the tubal ostium;
- Press the deployment button;
- Rotate the thumbwheel towards yourself again until it can no longer rotate (the micro-insert will unwind and detach from the delivery system);
- Remove the device delivery system;
- Count and record the number of expanded coils extruding from the tubal ostium -- ideally, according to the manufacturer, there should be three to eight expanded coils trailing into the uterus. If there are 18 or more expanded coils, remove the micro-insert and re-attempt insertion with a new device;
- Repeat the procedure at the contralateral tubal ostium.
After placement of the second micro-insert, the procedure is complete and the patient may be discharged home or to the post-anesthesia care unit. Other transcervical procedures can be performed with transcervical sterilization (e.g. endometrial ablation, endometrial polypectomy). However, a concomitant procedure should be avoided if it will cause bleeding that obscures the view of the tubal ostia or if electrocautery is used after the micro-inserts have been placed. Ablation methods that use types of energy that can conduct through the micro-inserts and cause tissue injury should not be used with the micro-inserts. Ablation methods that have been evaluated in various studies include hot water filled balloon and bipolar radiofrequency (9). There are no data regarding cryoablation techniques or use of laser. A cautious approach should be taken when combining ablation and hysteroscopic sterilization, since the Essure® manufacturer has issued a warning against concomitant Thermachoice® ablation and Essure® sterilization. This statement was based on an unpublished study in which 5 of 30 women who underwent the two procedures concurrently had severe intrauterine synechiae that precluded assessment of tubal patency.
n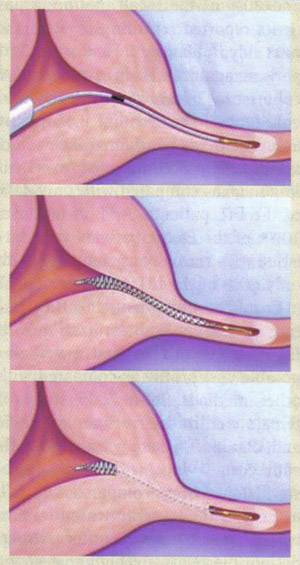
Micro-insert placement. The Essure® (Conceptus Inc., Mountain view, CA) procedure for permanent birth control
Follow-up and Confirming Occlusion:
Many women can return to their daily activities on the day of the procedure. Counsel patients to call for fever, excessive vaginal bleeding, severe or persistent pain or if they pass a micro-insert. Remember to advise patient to use another method of contraception until correct micro-insert location and bilateral tubal occlusion are confirmed. At 12 weeks after procedure, perform hysterosalpingogram (HSG) to confirm correct placement and bilateral tubal occlusion. In the United States, HSG is the Food and Drug Administration (FDA) approved standard for confirming correct micro-insert placement and bilateral tubal occlusion. A potential pitfall of hysteroscopic sterilization is the apparently low rate of compliance with post-operative HSG, 12 to 71% (10). Since HSG are costly, time-consuming and uncomfortable, some experts use pelvic radiography or transvaginal ultrasound as an initial test for appropriate placement, with HSG reserved for patients in whom unsatisfactory placement is suspected. However, this practice is controversial. The 12 week follow-up period is based on early efficacy trials when the hysteroscopic sterilization device was tested in a small cohort of subjects undergoing subsequent hysterectomy. There are few data regarding a shorter interval to tubal occlusion (11).
n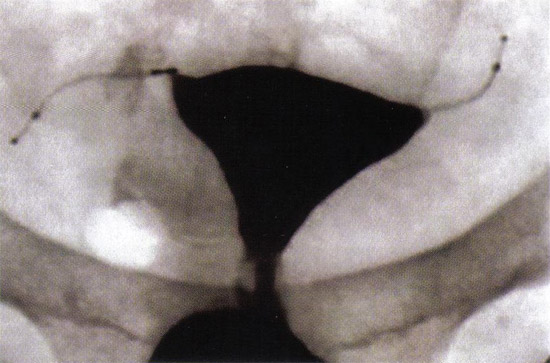
Tubal Occlusion is confirmed at 12 weeks following Essure® (Conceptus, Inc., Mountain View, CA) microinsert placement by hysterosalpingogram.
Safety and Effectiveness:
There are few long-term follow-up data regarding hysteroscopic sterilization. The longest follow-up study is a case series in which 163 women completed five years follow-up; no adverse device-related events were reported and 99% were tolerating the device well (12). Tubal and uterine perforation have been reported and may be asymptomatic or present as pelvic pain; some perforations are noted when a patient has post-procedure pregnancy. A review of the FDA's Manufacturer and User Facility Device Experience database from the introduction of Essure® in 2002 to July 2008 did not reveal any major adverse events (death, bowel injury, or major vascular injury), but there were 2 reports of devices embedding into abdominal structures and requiring removal after procedures complicated by uterine perforations. For women with significant medical problems (such as severe cardiac disease) who require permanent contraception but might otherwise carry considerable surgical risks, Essure® has been shown to be a safe alternative to tubal ligation. Expulsion of one or both micro-inserts has been reported. As an example, in one large series (n=1630), at up to 42 months of follow-up, the rate of expulsion of one micro-insert was less than 1% (12). Reports of persistent pelvic pain and infection are rare. Cornual abscess have been described in a woman who underwent hysteroscopic sterilization combined with endometrial ablation, although this is likely a very rare complication. No device related obstetric complications have been reported in women who have given birth after hysteroscopic sterilization.
Patient satisfaction is high after hysteroscopic sterilization. Outcome measures for this method include correct placement of device and contraceptive effectiveness. According to the phase II multicenter trial of effectiveness, no pregnancies were reported in 6015 woman-months of exposure to intercourse following documented bilateral tubal occlusion (13). In the two largest Essure® case series 10 pregnancies were reported after approximately 6,000 procedures and 64 pregnancies were reported after approximately 50,000 procedures. Of note, in both series, the number of procedures was estimated by the device distributor and the pregnancy rate was based on voluntary reporting to the distributor or study author. The most common reasons for contraceptive failure of hysteroscopic sterilization are: pregnant at the time of placement, incorrect placement -- unilateral or tubal or uterine perforation; non-compliance with postoperative instruction -- failure to use contraception until confirmation of occlusion or failure to have follow-up imaging; misreading of imaging study used to confirm bilateral occlusion.
The rate of successful placement of the Essure® permanent birth control device in a large study (14) at the university medical centers is 92.1%, with a post-Essure® pregnancy rate of 0.95%. The majority of placement failures may be attributed to difficulty visualizing the tubal ostia. Essure® hysteroscopic sterilization appears to provide a minimally invasive, practical and effective method of permanent sterilization.
Post-Procedure Restrictions:
Essure® micro-inserts are metallic, and thus patients should be counseled about the following post-procedure issues (15):
- Procedures involving pelvic use of unipolar electrocautery are contraindicated;
- Magnetic resonance imaging (MRI) is safe using a 1.5 tesla magnet (according to manufacturer); however, the micro-inserts produce an artifact, which will obscure imaging of local tissue.
Future Possibilities
Adiana:
The Adiana® (Hologic, Inc., Bedford, MA) sterilization method is a combination of controlled thermal damage to the lining of the fallopian tube followed by insertion of a non-absorbable biocompatible silicone elastomer matrix within the tubal lumen. Under hysteroscopic guidance, a delivery catheter is introduced into the tubal os. Once placement inside the intramural section of the fallopian tube is confirmed, the distal tip of the catheter delivers radiofrequency (RF) energy, causing a lesion within the fallopian tube. Following thermal injury, the silicone matrix is deployed in the region of the tube where the lesion was formed and the catheter and hysteroscope are removed. Over the next few weeks, occlusion is achieved by fibroblast in-growth into the matrix, which serves as permanent scaffolding and allows for "space-filling" (16). The mean procedure time is about 12 minutes. Patients require local anesthesia with occasional intravenous sedation. Occlusion of tubes is assessed by HSG 3 months after device placement.
Data from the pivotal study called the Evaluation of the Adiana System for Sterilization Using Electrothermal Energy (EASE) trial was presented to the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee for the FDA in December 2007. In this study, the primary endpoint was to demonstrate the effectiveness of the Adiana system (16). In this trial of 570 women, the cumulative failure rates were 1.08% at 1 year and 1.82 at 2 years. These effectiveness data suggest rates more than double that of laparoscopic tubal ligation and are further disappointing when evaluated vis-à-vis the data for Essure®. In the limited data available from the EASE trial, Adiana seems to be a relatively safe procedure. Postoperative pain after the procedure is reported in 25%, some cramping with procedure, which is done under local anesthesia in office setting, and only 2% complained of post-procedural pain. The costs associated with Adiana are as of yet unknown.
Quinacrine:
In 1973, Zipper and colleagues demonstrated the sclerosing effects of intrauterine quinacrine on the tubal ostia of rats. From these early animal experiments and the accepted clinical application of quinacrine for inducing pleural sclerosis, Zipper and associates proceeded to investigate the potential use of transcervical quinacrine as a method of sterilization in humans. In their initial trials, the quinacrine was delivered as slurry with dilutions of 125 mg/ml and 250 mg/ml. Despite promising early effectiveness results, this method was abandoned after 3 deaths were reported that were attributed to rapid absorption of the slurry through endometrial capillaries. Refining the technique, in 1977 Zipper and colleagues developed a new pellet-based method in which 7 pellets of 36 mg of quinacrine (252 mg total) are placed into the uterus using a tube similar to a copper T intrauterine device (IUD) inserter for 2 to 3 doses 1 month apart (17). Because of its profound implications for permanent contraception worldwide, quinacrine has been extensively studied, most frequently in developing countries where access to expensive medical technology is limited.
Quinacrine is a mutagen. It acts by chelation of DNA forming quinacrine-DNA complexes. Because of its role as a mutagen, it has been implicated as a potential carcinogen, although carcinogenicity in either humans or animals has never been established (17). Rather, since the 1930s, quinacrine has been used as an anti-malarial agent in over 100 million people without any associated increases in cancers. With respect to its potential as a teratogen, in over 130,000 quinacrine sterilizations worldwide there have been no reports of birth defects in any of the involved pregnancies. Finally, with the important exception of the 3 deaths reported with the early use of the quinacrine slurry, no deaths have been reported in over 130,000 cases using the pellets, whereas 3 to 10 deaths could have been expected in the same population undergoing tubal ligation in an industrialized country and perhaps 25 deaths in a less developed nation. In general, quinacrine sterilization is well tolerated. Major complications and side effects were not noted in Vietnamese trial (17). Only a small number of women were seen for problems such as salpingitis (3%), menstrual disorders (2.7%), and dysmenorrhea (2%). The most attractive feature of quinacrine sterilization form a world health perspective is cost. When quinacrine was manufactured by SIPHARM (Sisseln, Switzerland), the cost in Asia for the inserter and quinacrine pellets was less than $ 1 per sterilization. Compared with the cost of tubal ligation, the difference is dramatic and needs no further elaboration.
Cost Effectiveness:
The outpatient, in-office nature of transcervical sterilization with Essure® gives this method a very favorable cost profile as compared with other methods. This study compares the expected 5-year costs for permanent sterilization in women between non-incisional hysteroscopic tubal occlusion with the Essure® system performed in an office setting and laparoscopic bilateral tubal ligation (LBTL). An economic decision tree is used to predict outcomes and costs to compare these two procedures from a US Medicaid perspective over a 5-year time horizon. Expected costs are $2,367 for Essure® and $3,545 for LBTL (Essure® saves $1,178 or 33% of LBTL costs). Sensitivity analyses show Essure® has lower expected costs across all values considered. If the cost for a LBTL procedure were to decrease by 20% and the cost for Essure® to increase by 20%, Essure® would have still have lower expected costs. Office-based sterilization for women using Essure® can lead to substantial cost savings over 5 years compared to LBTL (18). This conclusion is robust to varying analytic inputs.
Summary:
After over 100 years of seeking a safe and effective method for female sterilization that avoids entry into the abdomen, transcervical sterilization is today a reality. Laparoscopic tubal ligation was considered the gold standard against which other methods for permanent female sterilization were judged. Almost all patients are candidates for this procedure, except for women with profound medical problems that preclude them from receiving general anesthesia, even for a short duration. Methods of tubal occlusion using electrocautery or Nd: YAG laser proved ineffective and were abandoned. Transcervical sterilization has proved effective and safe. To date, when all reported pregnancies with a confirmatory HSG are analyzed, hysteroscopic tubal occlusion represents the most effective of all female or male sterilization techniques at the observed follow-up times. The risk of pregnancy after hysteroscopic sterilization is approximately 0.1%. Reasons for failure include: pregnancy at the time of insertion, incorrect placement, non-compliance with postoperative instructions, and misreading of imaging study used to confirm bilateral occlusion. The Adiana® sterilization method is a combination of controlled thermal damage to the lining of the fallopian tube followed by insertion of a non-absorbable silicone elastomer matrix within the tubal lumen. Because of its profound implications for permanent contraception worldwide, quinacrine has been extensively studied, most frequently in poorer nations where access to expensive medical technology is limited.
References:
- Peterson HB. Sterilization. Obstet Gynecol 2008;111:189-203
- Johns DA. Advances in hysteroscopic sterilization: report on 600 patients enrolled in the Adiana EASE trial. J Minim Invas Gynecol 2005;12:S39-S40
- Arjona JE. Satisfaction and tolerance with office hysteroscopic tubal sterilization. Fertil Steril 2008;90:1182-1189
- Cooper JM, Carigan CS, Cher D et al. Microinsert non-incisional hysteroscopic sterilization. Obstet Gynecol 2003;102:59-67
- Valle RF, Carigan CS, Wright TC et al. Tissue response to the STOP micro-coil transcervical permanent contraceptive device: results from a pre-hysterectomy study. Fertil Steril 2001;76:974-980
- Essure [package insert]. Mountain View, CA: Conceptus, Inc.; 2007 www.essuremd.com/ Accessed September 16, 2009
- Kerin JF, Cattanach S. Successful pregnancy outcome with the use of in vitro fertilization after Essure hysteroscopic sterilization. Fertil Steril 2007;87:1212-1219
- Mino M, Arjona JE, Cordon J et al. Success rate and patient satisfaction with the Essure sterilization in an outpatient setting: a prospective study of 857 women. BJOG 2007;114:763-770
- Hopkins MR. Radiofrequency global endometrial ablation followed by hysteroscopic sterilization. J Minim Invas Gynecol 2008;14:494-501
- Shavell VI, Abdallah ME, Diamond MP et al. Post-Essure hysterosalpingography compliance in a clinic population. J Minim Invas Gynecol 2008;15:431-434
- Connor V. Contrast infusion sonography in the post-Essure setting. J Minim Invasive Gynecol 2008;15:56-61
- Ory EM, Hines RS, Cleland WH et al. Pregnancy after micro-insert sterilization with tubal occlusion confirmed by hysterosalpingogram. Obstet Gynecol 2008;111:508-510
- Levy B, Levie MD, Childers ME. A summary of reported pregnancies after hysteroscopic sterilization. J Minim Invas Gynecol 2007;14:271-274
- Shavell VI, Abdallah ME, Diamond MP et al. Placement of a permanent birth control device at a university medical center. J Rerpod Med 2009;54:218-222
- Wittner MH, Brown DL, Hartman RP et al. Sonography, CT, and MRI appearance of the Essure micro-insert permanent birth control device. Am J Roentgenol 2006;187:959-963
- US Food and Drug Administration Obstetrics and Gynecology Devices Panel. Adiana Transcervical Sterilization System PMA P070022 Panel Pakage, - pg 13-15, OB-GYN, December 14, 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4334b1-00-index.html Accessed September 15, 2009
- Sokal DC, Hieu do T, Loan ND et al. Safety of quinacrine contraceptive pellets: results from 10-year follow-up in Vietnam. Contraception 2008;78:66-72
- Kraemer DF, Yen PY, Nichols M. An economic comparison of female sterilization of hysteroscopic tubal occlusion with laparoscopic bilateral tubal ligation. Contraception 2009;80:254-260
Published: 23 November 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


