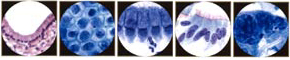
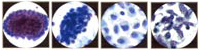
Pathogenesis of Cervical Adenocarcinoma
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Regular cervical screening has had a significant impact on the incidence and mortality associated with cervical cancer. The goal of screening in the USA is to identify precancerous lesions so they can be removed prior to progression of invasive cancer. The combined use of cytology and HPV DNA testing increases sensitivity but decreases specificity (1). In March 2003, the US Food and Drug Administration approved the Hybrid Capture 2 HPV DNA test (HC2) for use in the primary cervical screening of women aged 30 years and above when used in combination with cervical cytology. Hence, the combination of HPV testing and cervical cytology provide not only greater reassurance that cervical pre-cancer and cancer have not been missed, but also predict the level of risk for the future, which is not obtainable through screening with cytology alone.
The purpose of this document is to review cytological screening, DNA testing procedures and pathological features of glandular cells abnormalities. Consensus guidelines are available for the management of women with cervical cytological abnormalities and cervical cancer precursors. These evidence-based guidelines were developed in 2001 by an expert consensus conference sponsored by the American Society for Colposcopy and Cervical Pathology. Vaccines are currently being developed to reduce susceptibility to HPV infection and persistent infection. Widespread acceptance of these vaccines should significantly reduce the incidence of HPV-associated disease, thereby alleviating a significant fraction of morbidity associated with HPV infections.
Guidelines for cervical cytology:
The American Cancer Society (ACS) and the American College of Obstetricians and Gynecologists (ACOG) endorse either the conventional Pap test or liquid-based preparation (LBP) for cytological screening. The ACOG recommends the following practices to optimize cervical cytology (2): cells should be collected before bimanual examination, and care should be taken to avoid contaminating the sample with lubricant. If cervical samples are to be collected to test for sexually transmitted diseases (STDs), cell collection for cervical cytology should be undertaken first. Ideally, the entire portion of the cervix should be visible when the sample is obtained. Routine swabbing of the discharge from the cervix may result in cytological samples of scant cellularity. In an effort to reduce air drying artifact, the specimen should be transferred and fixed as quickly as possible. A 'satisfactory' specimen for cytological analysis has been defined by the Bethesda 2001 Workshop, a consensus workshop convened by the National Cancer Institute and co-sponsored by 44 professional societies (3). There should be at least 8,000-12,000 well-visualized squamous cells for conventional smears and 5,000 squamous cells for liquid-based preparations. There should be at least 10 well-preserved endocervical or squamous metaplastic cells. A specimen is considered 'partly obscured' when 50-75% of epithelial cells cannot be visualized; specimens with more than 75% of epithelial cells obscured are 'unsatisfactory'.
Glandular cell abnormalities less severe than adenocarcinoma may be classified into the following categories: atypical glandular cells (AGC), either endocervical, endometrial, or glandular cells not otherwise specified; atypical glandular cells (AGC) and endocervical adenocarcinoma in situ (AIS). The term 'atypical epithelial cells' may be used for cases where a squamous vs a glandular origin cannot be determined (4). An intermediate category 'atypical endocervical cells, favor neoplastic' and 'atypical glandular cells of undetermined significance, probably neoplastic' apply to cases showing some features suggestive of, but not sufficient to reach an interpretation of, adenocarcinoma in situ (AIS).
Glandular Cells:
This section includes benign endometrial cells, atypical glandular cells of undermined significance (AGUS) derived from either endocervical or endometrial cells, and adenocarcinomas (originating in the endocervix, endometrium, or extrauterine area). The "glandular" or columnar epithelium of the cervix is located cephalad to the squamo-columnar junction. It covers a variable amount of the ectocervix and lines the endocervical canal. It is comprised of a single layer of mucin-secreting cells. The epithelium is thrown into longitudinal folds and invaginations that make up the so-called endocervical glands (they are not true glands). These infolding crypts and channels make the cytologic and colposcopic detection of neoplasia less reliable and more problematic. The complex architecture of the endocervical glands gives the columnar epithelium a papillary appearance through the colposcope and a grainy appearance upon gross visual inspection. The single cell layer allows the coloration of the underlying vasculature to be seen more easily. Therefore, the columnar epithelium appears redder in comparison with the more opaque squamous epithelium.
Cytology of the Glandular Epithelium: The glandular epithelium of the female genital tract includes the lining of the endocervix, endometrium, and fallopian tube. Pap smear is not nearly as good a screening test for glandular lesions as it is for squamous lesions. Endocervical cells are tall and columnar, and can be secretory or ciliated. Endocervical cells can be seen singly or in strips or sheets. See pictures below:
Endometrial cells can also form three-dimensional clusters of glandular cells, without central stroma. The cells are small and crowded, and the nuclei are usually degenerated and hyperchromatic can mimic carcinoma in situ. A common everyday problem in Pap smear diagnosis is distinguishing endometrial cells from endocervical cells. This can be important, since shedding of endometrial cells is abnormal in the second half of the menstrual cycle (especially past 40 years of age) or any time in postmenopausal women. Abnormal shedding of endometrial cells carries with it an increased risk of endometrial hyperplasia or neoplasia. See picture below:
Benign Endometrial Cells - benign out-of-cycle endometrial cells are excluded from the Bethesda System, while those from postmenopausal women not on exogenous hormones must be explained. Endometrial cells may be present when dislodged from the lower uterine segment by endocervical brushing. They also may be seen because of benign polyps or hyperplasia. Occasionally, however, they are shed secondary to hormone producing neoplasms of the tubes or ovaries.
Atypical Glandular Cells of Undetermined Significance (AGUS) - the definition of AGUS according to the Bethesda System, is cells showing either endometrial or endocervical differentiation displaying nuclear atypia that exceeds obvious reactive or reparative changes but lacks unequivocal features of invasive adenocarcinoma. The diagnosis should be qualified if possible to indicate whether the cells are thought to be of endocervical or endometrial origin. Atypical endocervical cells may be sub-classified further according to whether a reactive or neoplastic process is favored. Criteria for separating atypical endometrial cells are not well defined and therefore this category is not further subdivided (5).
Atypical Endometrial Cells - cytoplasmic borders are ill defined, and there may be vacuoles. Nuclear changes include enlargement, hyperchromasia, and micronucleoli.
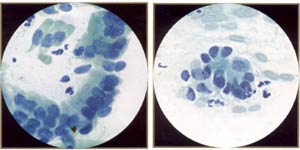 |
|
AGUS (atypical endocervical cells). These endocervical cells are somewhat hyperchromatic, crowded, and stratified, features that may suggest endocervical glandular neoplasia. However, these cells represent robal metaplasia. Note round, rather than elongated, nuclei and bland, rather than coarse, chromatin. Also note ciliated cells. |
AGUS (atypical endocervical cells). These endocervical cells are forming a microacinar structure, a feature that may suggest endocervical glandular neoplasia. However, these cells also represent tubal metaplasia, with bland, round nuclei. |
Atypical Glandular Cells - Comparative Cytomorphology:
| Distinctive Features | Reactive | LSIL | HSIL | Adenocarcinoma |
|---|---|---|---|---|
| Structure | Monolayered | Crowded | Piled | Multilayered |
| Cytoplasm | Defined | +/- | +/- | +/- |
| Nuclei: 1. Contour 2. Chromatin | Smooth Fine | Smooth Smudgy | Irregular Coarse | Irregular Clumped |
| Nucleoli | Macro | +/- | Micro/Macro |
Cervical adenocarcinoma in situ (AIS):
The relationship between AIS and lesser degrees of cervical glandular neoplasia is more controversial. There is convincing evidence that AIS is a precursor lesion. The mean age of diagnosis of AIS is 15 years younger than that for invasive adenocarcinoma. AIS frequently coexist with invasive adenocarcinoma in histologic specimens. The Bethesda System includes a category for abnormal glandular cells of undetermined significance (AGUS). Patients with AGUS smear reports have a 30% to 50% risk of having high-grade cervical disease. The underlying lesion is most frequently high-grade CIN, which occurs in up to 25% of patients, AIS, cervical adenocarcinoma, and endometrial disease, including hyperplasia and carcinoma, occur up to 20% of patients (6). Cytologic atypia (manifested by nuclear hyperchromasia and pleomorphism) and increased mitotic figures are the two most important identifying features. The presence of numerous apoptotic bodies is another diagnostic clue. The mucins secreted by the in situ malignant glands may be similar to those of the normal endocervical mucosa or may resemble the pattern of intestinal goblet cells. Most lesions are positive for CEA, less than half keratin, and one tenth for secretory component, those figures being lower than for invasive tumors. An important differential diagnosis of in situ cervical adenocarcinoma is with tubal or tubal-endometrial hyperplasia, further complicated by the fact that a tubal type of in situ endocervical adenocarcinoma has been described.
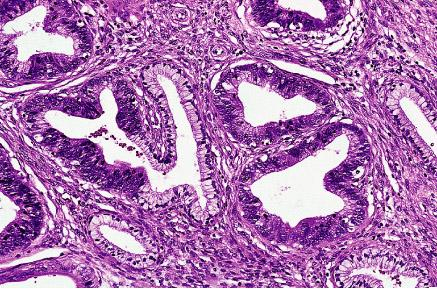
Adenocarcinoma in situ with partial preservation of endocervical glands
Adenocarcinoma:
Microinvasive cervical adenocarcinoma: It has been defined in lines similar to those previously agreed upon for microinvasive squamous cell carcinoma (7). Terms such as "early stage" and "early invasive" have also been used. The usual criterion used for its recognition is the presence in an in-situ-adenocarcinoma, of a focus of stromal invasion not exceeding 5 mm in depth. The treatment is dependent upon the horizontal extension and whether vascular invasion is present or absent.
Morphologic variants of cervical adenocarcinoma
Endometrioid adenocarcinoma: It closely resembles its endometrial and ovarian counterparts. It can be extremely well differentiated, and can be seen in association with a synchronous or metachronous endometrioid carcinoma of ovary. The incidence of this tumor type seems to be on the rise. The differential diagnosis includes endometrioid adenocarcinoma of the endometrium, which can be accompanied by a very deceptive pattern of cervical spread.
Papillary serous carcinoma: It is analogous in appearance to the homonymous uterine and ovarian neoplasm, including the common occurrence of psammoma bodies.
Adenoma malignum: It constitutes approximately 1% of all endocervical adenocarcinomas. Some cases are associated with the Peutz-Jeghers syndrome, and mutations of the STK11 gene (a tumor suppressor gene responsible for this syndrome) have been found in over half of the cases of this tumor type. It is a type of cervical adenocarcinoma differentiated structurally and cytologically that it can be diagnosed only as malignant because of the presence of distorted glands with irregular outlines deeply positioned in the cervix and the fact that a portion of the infiltrating tumor is associated with a stromal response. In addition, about half of the cases have small foci with a less well differentiated appearance. Vascular and perineurial invasion may be present. The mucin produced is predominantly of neural type. Adenoma malignum usually lacks evidence of high-risk HPV and p53 mutation (8).
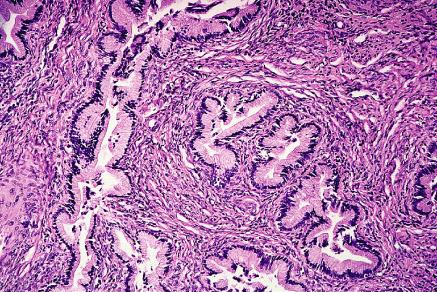
So-called "adenoma malignum" (minimal deviation adenocarcinoma) of cervix
Adenosquamous (mixed) carcinoma: It combines the patterns of adenocarcinoma with a well-defined squamous component. This tumor type seems to be particularly common during pregnancy. It is likely that the cell of origin of adenosquamous carcinomas and most adenocarcinomas of the cervix is the same as for the ordinary squamous cell carcinoma (eg, the subcolumnar reserve cell). Adenocarcinoma as previously defined should be distinguished from squamous cell carcinoma without glandular formations but with histochemically demonstrable intracellular mucin. Adenosquamous carcinomas have been shown in some series to have a worse overall prognosis than pure squamous cell carcinoma or adenocarcinoma, at least in advanced-stage lesions. This is probably due to the fact that most of them are poorly differentiated tumors, as supported by the greater proportion of high-ploidy stem cells found in these lesions when subjected to DNA analysis. However, when compared grade by grade and stage by stage with adenocarcinomas and squamous cell carcinomas, no prognostic differences are found (9).
Glassy cell carcinoma: It has been described as a distinct type of poorly differentiated adenosquamous carcinoma. It occurs in a younger age group (mean age, 41 years) than other cervical neoplasms and often has been associated with pregnancy. The tumor cells have a moderate amount of cytoplasm with a ground glass or finely granular appearance, a prominent eosinophilic and PAS-positive cell wall, and large nuclei with prominent nucleoli. Mitoses are numerous. A prominent inflammatory infiltrate, often rich in eosinophils, is regularly seen in the adjacent stroma, and this may be accompanied by peripheral blood eosinophilia. In pure cases of glassy cell carcinoma, glandular or squamous differentiation is absent, although it can be consistently detected by ultra-structural examination. The prognosis is poor, a fact probably related to its poorly differentiated nature.
Villoglandular (papillary) adenocarcinoma: It presents as an exophytic polypoid lesion with papillae lined by endocervical, endometrial, or intestinal-type epithelium showing mild atypia. The appearance of the superficial portion is similar to that of colorectal villous adenoma. Most cases are associated with adenocarcinoma in situ and/or CIN, and there is usually evidence of HPV infection. The prognosis is excellent.
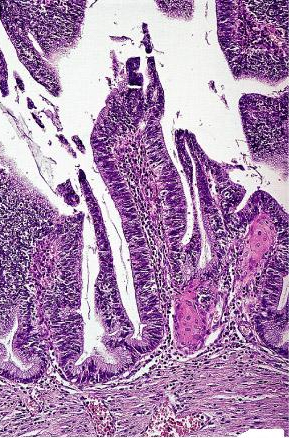
Villoglandular variant of endocervical adenocarcinoma; the architecture is reminiscent of colorectal villous adenoma
Adenoid basal carcinoma: It should be distinguished both from adenoid cystic carcinoma and from basaloid (squamous cell) carcinoma. Although it shares many phenotypical features with adenoid cystic carcinoma, it is a very low-grade lesion, in contrast with the two neoplasms with which it can be confused. It is usually discovered incidentally, it does not produce a mass lesion, and it has never resulted in metastases. The consistent presence of HPV-16 has been documented. The lesion blends with the process that has been called adenoid basal hyperplasia. Brainard et al have proposed grouping these two conditions under the term adenoid basal epithelium to emphasize their indolent nature.
Adenoid cystic carcinoma: It is a specific variant of cervical adenocarcinoma that tends to occur in elderly multigravid black women and that is associated with a particularly poor prognosis. The morphologic appearance is similar to that of the homonymous tumors of salivary glands. Like the former, it may be cribriform (the most common pattern) or grow in a predominantly solid fashion.
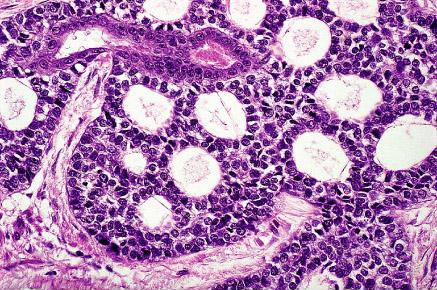
Adenoid cystic carcinoma of cervix with a typical cribriform pattern of growth
Clear cell carcinoma of cervix: It is of müllerian rather than of mesonephric origin. The presence of in situ changes in the area of the squamocolumnar junction in some of the cases and the electron microscopic features seem to provide conclusive evidence for this interpretation. Glands lined by large cells with abundant clear cytoplasm are characteristic. "Hobnail" cells are common, as are intraglandular papillary projections. Grossly, the tumor is usually exophytic. This is the most common form of cervical carcinoma in young females, although it occurs in all age groups, and it shows a second peak at age 70 years. The prognosis is relatively good. The relationship with intrauterine diethylstilbestrol exposure and other features of this tumor are the same as for the analogous vaginal neoplasms. It is also evident, however, that morphologically identical cases occur in the absence of exposure to this hormone, particularly in older women.
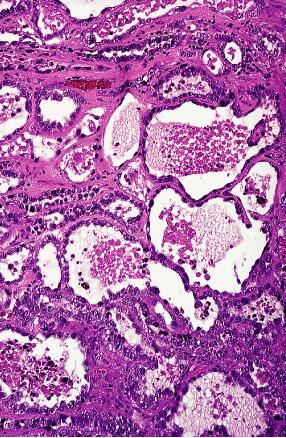
Clear cell adenocarcinoma of cervix showing tubular, microcystic, and tubulocystic features
Mesonephric (adeno) carcinoma: It is a very rare tumor. Most of the cases that have been reported as such in the past probably represent müllerian-type adenocarcinomas or yolk sac tumors. True mesonephric carcinomas are often found adjacent to mesonephric hyperplasia (sometimes florid and atypical) and may exhibit a variety of patterns, such as ductal (resembling endometrioid adenocarcinoma), small tubular, retiform, solid, sex-cord-like, and spindle. The immunehistochemical profile is similar to that of mesonephric rests, and includes positivity of CD10 and calretinin, two interesting but not entirely specific findings.
Other variants: Such as endocervical adenocarcinoma, all of them exceedingly rare, are a micro-cystic type, a type associated with Paget's disease, a signet ring type, a small intestinal (enteric) type, and a type with choriocarcinomatous and hepatoid differentiation.
Summary:
AIS is the recognizable precursor to invasive adenocarcinoma. Unlike squamous cell carcinoma, earlier neoplastic precursors (low-grade or high-grade lesions) to AIS and adenocarcinoma are not well-characterized. The duration of progression from AIS to adenocarcinoma has been estimated to be 5-13 years. AIS usually originates in the squamocolumnar junction of the transformation zone and may extend well up into the endocervical canal. It has been postulated that oncogenic virus types infect reserve cells of the transformation zone that are committed to glandular differentiation, which eventually leads to the proliferation of atypical glandular cells (AGC) and AIS (10). Conventional Papanicolaou screening is not as sensitive in the detection of AGC or AIS, which leads to adenocarcinoma. Improved likelihood of obtaining abnormal glandular cells from the endocervix and higher regions of the transformation zone can be achieved through the use of endocervical brush after sampling of the ectocervix and transformation zone with an extended-tip spatula.
Acknowledgment: Women's Health and Education Center (WHEC) expresses gratitude to Dr. Bradley J. Monk, Associate Professor and Director of Research, Division of Gynecology Oncology, University of California Irvine Medical Center, California (USA) for his priceless assistance in developing the manuscript. We are looking forward to collaborate with him for many years to come. This information is designed to aid healthcare providers in making decisions about appropriate care of obstetric and gynecologic care. Variations in practice may be warranted based on the needs of the individual patient, resources, and limitations unique to the institution or type of practice.
References:
- Mandelblatt JS, Lawrence WF, Womack SM, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA 2002;287:2372-2381
- ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, no. 45, August 2003. Cervical cytology screening (replaces committee opinion 152, March 1995). Obstet Gynecol 2003;102:417-427
- Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-2119
- Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline of the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002;52:342-362
- Zaino RJ. Symposium part I: adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 2002;21:314-326
- Sharpless KE, Schnatz PF, Mandavilli S, et al. Dysplasia associated with atypical glandular cells on cervical cytology. Obstet Gynecol 2005; 105:494-500
- Bai H, Sung CJ, Steinhoff MM. Thin-Prep Pap test promotes detection of glandular lesions of the endocervix. Diagn Cytopathol 2000;23:19-22
- Koh WJ, Paley PJ, Comsia ND, et al. Radical management of recurrent cervical cancer. In: Eifel PJ, Levenback c, eds. American Cancer Society atlas of clinical oncology: neoplasms of the female genital tract. Hamilton, Ontario: BC Decker; 2001 (Level III)
- Rosi J. Morphologic variants of cervical adenocarcinoma. In: Rosai and Acherman's Surgical Pathology. Ninth edition; 2004
- Herzog TJ, Monk BJ. Reducing the burden of glandular carcinomas of the uterine cervix. Am J Obstet Gynecol 2007;197;566-571
Published: 2 December 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com