Placental Abnormalities & Major Obstetric Hemorrhage
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers.
Educational grant provided by Women's Health and Education Center (WHEC).
Bleeding in the second half of pregnancy and in labor due to placental abnormalities include placenta previa, abruptio placentae, placenta accreta and vasa previa. Third-trimester bleeding complicates about 3.8% of all pregnancies. Placenta previa is documented in 22% of cases, and strong evidence of abruptio placentae is found in 31%. In the remaining 47% of cases, the bleeding can be ascribed either to early labor (so-called marginal separation) or local lesions of the lower genital tract, or no source can be identified. Therefore, third-trimester bleeding ultimately proves to be of little consequence in about half the cases, but in the other half it is potentially life-threatening. Vaginal bleeding in the third-trimester is alarming to the pregnant woman and usually prompts immediate consultation with the physician.
The purpose of this document is to present evidence-based approach to the management of placental abnormalities and major obstetric hemorrhage. Attention to improving the hospital systems is necessary for the care of women at risk for major obstetric hemorrhage. It is important in the effort to decrease maternal mortality from hemorrhage. Multidisciplinary team implementation systemic changes are also discussed. It is the responsibility of the physician to decide without delay whether the cause is benign or potentially life-threatening to the mother, fetus, or both. The potential harm from either procrastination or unnecessary intervention may be extreme.
Placenta Previa
Placenta previa is defined as implantation of the placenta in the lower uterine segment in advance of the fetal presenting part. The placenta either totally or partially lies within the lower uterine segment. Placenta previa complicates approximately 0.3%-0.5% of pregnancies or about 4.8 per 1,000 deliveries. The risk of recurrent placenta previa is as high as 4% to 8%. The risk of placenta previa increases with the number of prior cesarean sections, rising to 10% with four or more. Although some distinctions in outcome may be made among the different degrees of true placenta previa, all are potentially associated with life-threatening hemorrhage during labor. The degree of placenta previa cannot alone predict the clinical course accurately, nor can it serve as the sole guide for management decisions. Thus, the importance of such classifications has diminished.
Traditionally, placenta previa has been categorized into 4 types (1):
- Complete placenta previa: where the placenta completely covers the internal os;
- Partial placenta previa: where the placenta partially covers the internal os. Thus, this scenario occurs only when the internal os is dilated to some degree;
- Marginal placenta previa: where placenta just reaches the internal os, but does not cover it;
- Low-lying placenta: where placenta extends into the lower uterine segment but does not reach the internal os.
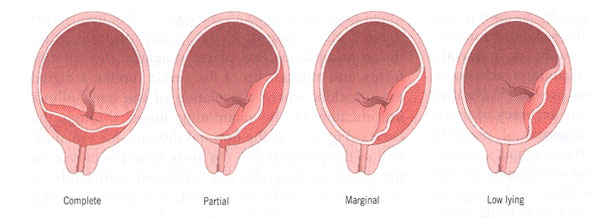
Types of placenta previa.
Pathophysiology:
The women at highest risk are those with prior placenta previa or multiple prior cesarean sections. The strong association between placenta previa and parity has suggested that "endometrial damage" is an etiologic factor. Presumably, each pregnancy "damages" the endometrium underlying the implantation site, rendering the area unsuitable for implantation. Subsequent pregnancies are more likely to become implanted in the lower uterine segment by a process of elimination. This effect is most clearly seen with prior term pregnancies, but multiple early pregnancy terminations may also be related to an increased incidence of placenta previa. There is evidence that low implantations are much more common early in pregnancy, but that the great majority of these "resolves" and never become symptomatic. With the progression of pregnancy, more than 90% of these low-lying placentas identified early in pregnancy will appear to move away from the cervix and out of the lower uterine segment. Although the term "placental migration" has been used, most authorities do not believe the placenta moves. Rather, it is felt the placenta grows preferentially toward a better vascularized fundus (trophotropism), whereas the placenta overlying the less well vascularized cervix may undergo atrophy (2).
Subsequent growth of the placenta after low implantation is either centripetal (resulting in central placenta previa) or unidirectional toward the more richly vascularized fundus. The latter mechanism is common, as demonstrated by the finding of an eccentric, marginal, or even velamentous insertion of the cord. The association of velamentous cord insertions with placenta previa and the pathologic entity of vasa previa are both consistent with a dynamic process sometimes called "placental migration". Unidirectional growth of the placenta coupled with disappearance of the early placenta at the original implantation site results in a placenta that appears to have moved away from its original location. The insertion point of the cord on the membranes marks the original location of the definitive placenta. The primary implantation site is probably low in the great majority of cases. An alternative mechanism involving fundal implantation with unidirectional growth toward the cervix has been suggested, but this mechanism has been observed only rarely with serial sonograms. Therefore, a fundal placenta in the second trimester is reassuring evidence that a placenta previa will not exist in the third trimester.
Diagnosis:
The classic clinical presentation of placenta previa is painless bleeding in the late second trimester or early third trimester. However, some patients with placenta previa will experience painful bleeding, possibly the consequence of uterine contractions or placental separation, whereas others will experience no bleeding at all before labor. Placenta previa may also lead to an unstable lie or malpresentation in late pregnancy. The majority of cases of placenta previa are diagnosed during routine sonography in asymptomatic women, usually during the second trimester. The initial episode of bleeding has a peak incidence at about the 34th week of pregnancy, although one-third of cases become symptomatic before the 30th week and one-third after the 36th week. Absence of bleeding prior to term does not rule out placenta previa. In approximately 10% of cases, bleeding begins only with the onset of labor, and in these situations one is more likely to find a partial or marginal placenta previa, or a low-lying placenta. Although transabdominal sonography is frequently used for placental location, this technique lacks some precision in diagnosing placenta previa. Numerous studies have demonstrated the accuracy of transvaginal sonography for the diagnosis of placenta previa, uniformly finding that transvaginal sonography is superior to transabdominal sonography for this finding. False-positive and --negative rates for the diagnosis of placenta previa using transabdominal sonography range from 2% to 25%.
Transvaginal imaging technique if used properly does not lead to increase in bleeding (3). This is for 2 main reasons: the vaginal probe is introduced at an angle that places it against the anterior fornix and anterior lip of the cervix, unlike a digital examination, where articulation of the hand allows introduction of the examining finger through the cervix; and the optimal distance for visualization of the cervix is 2-3 cm away from the cervix, so the probe is generally not advanced sufficiently to make contact with the placenta. Nonetheless, the examination should be performed by personnel experienced in transvaginal sonography, and the transvaginal probe should always be inserted carefully, with the examiner looking at the monitor to avoid putting the probe in the cervix. Translabial sonography has been suggested as an alternative to transvaginal sonography and has been shown superior to transabdominal sonography for placental location. However, because transvaginal sonography is accurate, safe and well tolerated, it should be the imaging modality of choice.
Management:
The two major factors have been responsible for the dramatic reduction in both maternal and perinatal mortality rates over the past 40 years: the expectant management approach and the liberal use of cesarean section rather than vaginal delivery. As a result, the maternal mortality rate has fallen from between 25% and 30% to less than 1%. The total perinatal mortality rate has fallen from between 60% and 70% to under 10% in the past 10 years. The goal of management for placenta previa is to obtain the maximum fetal maturation possible while minimizing the risk to both the fetus and the mother. The basis for this approach is that episodes of bleeding are usually self-limited and not fatal to either the fetus or the mother in the absence of inciting trauma (e.g., intercourse, pelvic examination) or labor. Under carefully controlled conditions, delivery of the fetus may be safely delayed to a more advanced stage of maturity in a significant proportion of cases. An additional advantage to this approach is that a small proportion of cases, particularly those discovered early with lesser degrees of placenta previa, will resolve to an extent permitting vaginal delivery at term. It is reasonable to hospitalize women with placenta previa while they are having an acute bleeding episode or uterine contractions. Women who present with bleeding in the second half of pregnancy should have a sonographic examination for placental location prior to any attempt to perform a digital examination. Digital examination with a placenta previa may provoke catastrophic hemorrhage and should not be performed.
It is reasonable to hospitalize women with placenta previa while they are having an acute bleeding episode or uterine contractions. One to two wide-bore intravenous cannulas should be inserted and blood taken for a full blood count and type and screen. In the absence of massive bleeding or other complications, coagulation studies are not helpful. The blood bank must be capable of making available at least 4 units of compatible packed red blood cells and coagulation factors at short notice. Rh immune globulin should be administered to Rh-negative women. A Kleihauer-Bettke test for quantification of fetal-maternal transfusion should also be performed in Rh-negative women because the mother may require increased doses of Rh immune globulin. Small studies have suggested a benefit of tocolytic therapy for women with placenta previa who are having contractions (4). Contractions may lead to cervical effacement and changes in the lower uterine segment, provoking bleeding, which in turn, stimulates contractions, creating a vicious cycle. Steroids should be administered in women between 24 and 34 weeks of gestation, generally at the time of admission for bleeding, to promote fetal lung maturation. The patient and her family should have a neonatology consultation so that the management of the infant after birth may be discussed. In women who have a history of cesarean delivery or uterine surgery, detailed sonography should be performed to exclude placenta accreta.
Before 32 weeks of gestation, moderate-to-severe bleeding when there is no maternal or fetal compromise may be managed aggressively with blood transfusions, rather than resorting to delivery. When the patient has had no further bleeding for 48 hours, she may be considered for discharge as long as there are appropriate home conditions to allow outpatient management. Women who are stable and asymptomatic, and who are reliable and have quick access to hospital, may be considered for outpatient management.
Timing of Delivery and Mode of Delivery:
As gestational age advances, there is an increased risk of significant bleeding, necessitating delivery. It is preferable to perform a cesarean delivery for placenta previa under controlled scheduled conditions rather than as an emergency. In a stable patient, it is reasonable to perform a cesarean delivery at 36-37 weeks of gestation, after documentation of fetal lung maturity by amniocentesis. If the amniocentesis does not demonstrate lung maturity and patient is stable it is reasonable to wait till 38 or 39 weeks of pregnancy or earlier if bleeding occurs or patient goes into labor. There is consensus that a placenta previa which totally or partially overlies the internal cervical os requires delivery by cesarean. However, the mode of delivery when the placenta lies in proximity to the internal os is more controversial. Women with a placenta -- internal os distance of less than 2 cm who undergo a trial of labor almost invariably experience significant bleeding during labor, necessitating cesarean delivery and many centers recommend cesarean delivery in these cases. Women whose placentas are 2 cm or more away from the os can undergo a normal labor. It is important to realize that, in women with a placenta that extends into the non-contractile lower uterine segment who have a vaginal delivery, there is potential for postpartum hemorrhage.
Anesthesia for delivery: in the past, it was generally recommended that cesarean deliveries for placenta previa be performed under general anesthesia. It was believed that this allowed more controlled surgery. Many studies have found these are associated with significantly greater estimated blood loss and greater requirements for blood transfusion than those performed under regional anesthesia, possibly due to increased uterine relaxation associated with general anesthetic (5). Many institutions generally perform cesarean deliveries for placenta previa under regional anesthesia.
Abruptio Placentae
The term abruptio placentae denote separation of a normally implanted placenta prior to the birth of the fetus. The diagnosis is most commonly made in third trimester, but the term may be used after the 20th week of pregnancy when the clinical and pathologic criteria are met. This is uniquely dangerous condition to both the mother and the fetus because of its pathologic sequelae. Placental separation is a serious complication of pregnancy. The reported incidence varies from 0.49% to 1.29% with a mean incidence of 0.83% or one per 120 deliveries.
Pathophysiology:
Abruptio placentae are initiated by bleeding into the decidua basalis. In most cases the source of the bleeding is small arterial vessels in the basal layer of the decidua that are pathologically altered and prone to rupture. The resultant hemorrhage splits the decidua, leaving a thin layer attached to the placenta. As the decidual hematoma grows there is further separation. Compression by the expanding hematoma leads to obliteration of the overlying inter-villous space. Ultimately there is destruction of the placental tissue in the involved area. This area may often be identified on gross inspection of the placenta by an organized clot lying within a cup-shaped depression on the maternal surface. From the standpoint of the fetus, this occurrence represents a loss of surface area for exchange of respiratory gases and nutrients. In a small number of cases, the process may be self-limited and of no further consequence to the pregnancy. If the initial separation is toward the center of the placenta there may be continued dissection and separation in the decidua as well as extravasation into the myometrium and through to the peritoneal surface. This results in the so-called Couvelaire uterus. Once the blood reaches the edge of the placenta it may continue to dissect between the decidua and the fetal membranes and gain access to the vagina through the cervix. It may pass through the membranes into the amniotic sac, causing the port wine discoloration that is almost pathognomonic of abruption. The amount of blood that eventually finds its way through the cervix is often only a small portion of that lost from the circulation, and in no way is a reliable indication of the severity of the condition.
Classification:
This classification is entirely retrospective, being assigned after delivery. A prospective classification, offering guidance for management, remains to be defined (6)
Grade | Concealed Hemorrhage | Uterine Tenderness | Maternal Shock | Coagulopathy | Fetal Distress | Comments |
| 0 | No | No | Absent | No | No | A retrospective diagnosis by examination of the placenta. Asymptomatic. |
| 1 | No | No | Absent | No | No | Includes the diagnosis of "marginal sinus rupture". Blood loss variable. |
| 2 | Yes | Yes | Absent | Rare | Yes | Will usually progress to grade 3 unless delivery is effected promptly. |
| 3 | Extensive | Yes | Present | Common | Fetal Death | Major maternal complication (e.g., renal cortical necrosis). |
Etiology:
Numerous factors have been suggested to play a causal role in abruptio placentae, but a unifying etiologic concept is still lacking. Some factors that have been suggested to play an etiologic role in the development of abruptio placentae are as follows: trauma; short umbilical cord or uterine anomaly; inferior vena cava compression; maternal hypertension; folic acid deficiency; cigarette smoking; maternal age and parity; cocaine abuse. Abruptio placentae is clearly not an "accident" in the great majority of cases, but rather an expression of a pathologic process of long duration. No better evidence exists than the risk of recurrence abruption in subsequent pregnancies. The risk of recurrence had been reported to be 5.5% to 16.6%, as much as 30 times the incidence in the general population.
Maternal Hypertension: mild abruption is not associated with clinically apparent hypertension. However, nearly 50% of cases of severe abruption with a dead fetus are associated with maternal hypertension, about half chronic and half pregnancy related; this rate represents a five-fold increase over the rate of hypertension in patients without abruption (7). These observations are consistent with the hypothesis that underlying maternal vascular disease is etiologic in both the hypertension and the abruption, and that abruption may be an even more sensitive indicator or vascular pathology than hypertension.
Diagnosis:
The classic symptoms and signs of abruptio placentae are vaginal bleeding, abdominal pain, uterine contractions, and uterine tenderness. The clinician must be aware that all of these are not invariably present and that the absence of one or more does not exclude the diagnosis or necessarily suggest a mild form. Ultrasonography is an important tool in the diagnosis of third-trimester bleeding. Its chief role is the diagnosis of placenta previa. The role of an ultrasonogram in the diagnosis of abruption is more problematic. The finding of a retroplacental sonolucency is consistent with retro-placental blood and is highly suggestive of placental abruption. However, a normal ultrasonogram of the placenta does not preclude the diagnosis of placental abruption. The sensitivity of ultrasonography has been reported as approximately 25%. Newer modalities under investigation include Doppler flow changes and thrombomodulin -- a marker of endothelial cell damage (8). Currently, however, placental abruption remains a clinical diagnosis. Heavy vaginal bleeding in a woman with hypertension, regular contractions, and no evidence of placenta previa render placental abruption the most likely diagnosis. The other diagnoses may be associated with vaginal bleeding, but they do not fit the clinical picture. Bleeding from vasa previa, if heavy, would produce fetal heart rate changes and would occur after the membranes had ruptured. Although bleeding from placenta accreta or succenturiate lobe may occur before delivery, such bleeding usually occurs in the third stage of labor.
Management:
Any patient with suspected abruptio placentae should be hospitalized immediately. A rapid evaluation of the mother's condition is made, including postural vital signs and abdominal examination. A blood pressure reading in the normal range may be misleading, because an underlying hypertensive condition may be revealed only after intravascular volume is restored. The vital signs are repeated at least every 15 minutes thereafter. If the fetus is alive, an external monitor is placed and evidence of fetal distress is sought. If the diagnosis of abruptio placentae is clinically favored over that of placenta previa, it is highly desirable to perform ultrasonographic examination in the labor and delivery suite. A large-bore intravenous line is placed, and through it the initial blood samples for initial hemoglobin and hematocrit, blood for type-screen and cross-match, baseline electrolyte and renal function studies are useful for later comparison when massive transfusion is required as well as for later detection of renal complications. Clinically significant coagulopathy is encountered in only about 10% of cases of abruption, but it is much more common in severe abruption marked by death of the fetus or massive hemorrhage. Proper management of this disorder demands an understanding of its pathophysiology as well as correct interpretation of various laboratory tests of hemostasis and blood coagulation.
Once diagnosis is established and cardiovascular resuscitation is well under way, a rational plan for delivery of the fetus must be done. This is the single most therapeutic goal. The method and timing of delivery depend on the condition and gestational age of the fetus, the condition of the mother, and status of cervix. Amniotomy is advantageous in nearly all cases. It probably reduces extravasation of blood into the myometrium and entry of thromboplastic substances into maternal circulation, and it may stimulate labor. A major advantage is that amniotomy allows placement of a fetal scalp electrode for heart rate monitoring and an intra-amniotic catheter. Numerous studies of abruption over the past 25 years have demonstrated improved perinatal survival with increased and early use of cesarean section for delivery. If the fetus is dead, vaginal delivery should be attempted in order to minimize maternal morbidity. The clinician must consider each case as unique in deciding the appropriate method of delivery. Meticulous attention to correct surgical technique is more important than "shotgun" prophylactic therapy for coagulopathy in avoiding major intraoperative and postoperative complications. Particular emphasis should be placed on ligation or cautery of small bleeding points that might be overlooked in the routine case.
Placenta Accreta
Vasa Previa
Vasa previa refers to fetal vessels running through the membranes over the cervix and under the fetal presenting part, unprotected by placenta or umbilical cord. The condition usually results either from a velamentous insertion of the cord into the membranes rather than the placenta or from vessels running between lobes of a placenta with one or more accessory lobe. It is a condition which, if undiagnosed is associated with a perinatal mortality of approximately 60%. The condition is important because, when the membranes rupture, spontaneously or artificially, the fetal vessels running through the membranes have a high risk of concomitant rupture, frequently resulting in fetal exsanguination and death. The incidence of vasa previa is approximately 1 in 2,500 deliveries. Risk factors for the condition include a second-trimester low-lying placenta (even if the "low lying" placenta or placenta previa resolves in the third-trimester), pregnancies in which the placenta has accessory lobes, multiple pregnancies, and pregnancies resulting from in vitro fertilization.
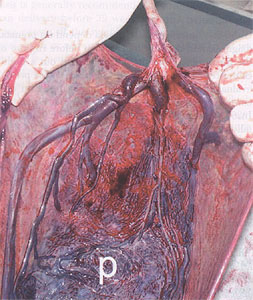
Placenta after delivery showing vasa previa. Vessels are seen running unprotected through the membranes.
Diagnosis and Management:
Vasa previa is most commonly diagnosed when rupture of the membranes is accompanied by vaginal bleeding and fetal distress or death. However, when acute bleeding occurs from a ruptured vasa previa, emergent delivery is frequently indicated, and there may be no time to test for fetal blood cells. Numerous reports and studies have demonstrated that vasa previa can be diagnosed prenatally with ultrasonography (9). When a color or power Doppler is used, flow can be demonstrated through these vessels, and pulsed Doppler will demonstrate a fetal umbilical arterial or venous waveform. It is important to differentiate a vasa previa from a funic presentation. In the latter, the vessels will move when the patient changes position, especially when the patient is placed in the Trendelenburg position. Conversely, the vessels do not move when there is a vasa previa. The majority of cases of vasa previa in asymptomatic women can be diagnosed prenatally through a policy of routinely evaluating the placental cord insertion when an ultrasound examination is performed and considering vaginal sonography with color Doppler if the placental cord insertion cannot be identified or if there is a low-lying placenta or a suspected succenturiate placental lobe. Screening for vasa previa should be routine in obstetric sonogram.
Good outcome with vasa previa depend on prenatal diagnosis and delivery by cesarean before rupture of the membranes. Consideration should be given to hospitalization at about 30-32 weeks and administration of corticosteroids to promote fetal lung maturation. Delivery should occur at an institution where there are adequate facilities for neonatal resuscitation that might include emergent blood transfusions. It is preferable, that before surgery, the surgeon is aware of the position of the fetal vessels and plans the incision to avoid lacerating these vessels. A gestational age of between 35 to 36 weeks is the optimal age for cesarean delivery in women with vasa previa, with a reasonable trade off between prematurity with the risk of respiratory distress syndrome and that of rupture of the membranes with the risk of fetal exsanguination and death.
Major Obstetric Hemorrhage & Improving Hospital Systems:
Attention to improving the hospital systems necessary for the care of women at risk for major obstetric hemorrhage is important in the effort to decrease maternal mortality from hemorrhage. Nationwide, major obstetric hemorrhage and the need for cesarean hysterectomy have increased in recent years. In the setting of intractable obstetric hemorrhage, emergency peripartum hysterectomy is used as a life-saving procedure. Maternal death is a known complication of major obstetric hemorrhage. The creation of a patient safety team that works to improve the hospital systems for caring for women at risk for major obstetric hemorrhage can help to identify and manage these situations and save lives. Development of clinical pathways, guidelines and protocols designed to provide early diagnosis of patients at risk for major obstetric hemorrhage and for streamlined care in emergency situations are essential. A multidisciplinary patient safety team that includes individuals from the Division of Obstetric Anesthesiology, Maternal Fetal Medicine, Neonatology, and the Blood Bank as well as Departments of Nursing, Communication, and Administration and quarterly mock drills of rapid response team, helps to respond to these situations effectively (10).
The suggested protocols and guidelines for rapid response team includes: 1) Patients with known placenta previa should have consultation with Maternal Fetal Medicine, senior gynecologic surgeons, and Obstetric Anesthesiologist, ultrasonography to identify placenta accreta, twice-weekly type and screen to allow for more rapid availability of blood products in the event of major hemorrhage; and planned cesarean section at 36 week after amniocentesis for fetal lung maturity. 2) Preparation for major hemorrhage in patients with suspected placenta accreta included weekly autologous blood donation; erythropoietin, iron, and vitamin therapy; possible consultation with interventional radiology, judicious placement of extra intravenous lines, and a 7.5 French internal jugular cordis for invasive monitoring and volume replacement; intraoperative monitoring with an arterial line and central venous pressure; and transfer to the post-anesthesia care unit or surgical intensive care unit as needed. 3) Peripartum and intraoperative consultation with the Trauma Team as necessary. 4) Patient counseling for cesarean hysterectomy when placenta accreta is suspected. 5) Scheduling cesarean section and cesarean hysterectomy in the main operating room under the direction of senior gynecologic surgeons, instead of labor and delivery unit. The response of major obstetric hemorrhage must be rapid and multifaceted to be successful. Principles of quality improvement require that "systems" thinking take place when confronted with adverse outcomes.
Acknowledgement: Gratitude is expressed to Dr. Yinka Oyelese for his insights and help in preparation of this manuscript. We at Women's Health and Education Center (WHEC) are grateful to him for his support, friendship and technical expertise provided to this e-learning project.
References:
- Oyelese Y, Smulian JC. Placenta Previa, Placenta Accreta, and Vasa Previa. Obstet Gynecol. 2006;107:927-941
- Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York (NY): Springer;2000
- Mustafa SA, Brizot ML, Carvalho MH et al. Transvaginal ultrasonography in predicting placenta previa at delivery: a longitudinal study. Ultrasound Obstet Gynecol. 2002;20:356-359
- Sharma A, Suri V, Gupta I. Tocolytic therapy in conservative management of symptomatic placenta previa. Int J Gynecol Obstet. 2004;84:109-113
- Hong JY, Jee YS, Yoon HJ et al. Comparison of general and epidural anesthesia in elective cesarean section for placenta previa totalis: maternal hemodynamics, blood loss and neonatal outcome. Int J Obstet Anesth. 2003;12:12-16
- Maternal.Fetal Medicine Principles and Practice. Editors Creasy RK and Resnik R. Third edition. 1994; pp 610, publisher W.B. Saunders Company.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22
- Arabin B, van Eyck J, Laurini RN. Hemodynamic changes with paradoxical blood flow in expectant management of abruptio placentae. Obstet Gynecol. 1998;91:796-798
- Canterino JC, Mondestin-Sorrentio M, Muench MV et al. Vasa previa: prenatal diagnosis and evaluation with 3-dimensional sonography and power angiography. J Ultrasound Med. 2005;24:721-725
- Skupski DW, Issac LP, Fredric WI et al. Improving hospital systems for the care of women with major obstetric hemorrhage. Obstet Gynecol. 2006;107:977-983
Published: 6 August 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


