Intrapartum Electronic Fetal Heart Rate Monitoring
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
By the start of the 20th century, auscultation of the fetal heart rate during labor was the predominant method of assessment, and it remained so for many decades. However, when electronic fetal monitoring (EFM) was introduced in the 1960s, the idea of receiving continuous data by EFM was thought to be superior with the expectation that its use would be valuable in diagnosing fetal acidemia, thereby improving outcomes by preventing fetal death and morbidity. Electronic fetal monitoring (EFM) was introduced in the 1960s based on an assumption of efficacy, but meta-analyses conducted in the 1990's clearly indicate that the use of EFM increases the rate of cesarean deliveries and operative vaginal deliveries without improving perinatal outcomes in healthy, term pregnancies without risk factors. As such, the Society of Obstetricians and Gynecologists of Canada (SOGC), the Royal College of Obstetricians and Gynecologists (RCOG, United Kingdom) and the Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG) recommend intermittent auscultation as the preferred method of intrapartum fetal surveillance for healthy, term women without risk factors for adverse perinatal outcome. EFM should be used for women with risk factors for adverse perinatal outcome or when intermittent auscultation findings are abnormal. In the United States, EFM was used among 45% of women in labor, in 1980, 62% in 1988, 74% in 1992, and 85% in 2002 (1) or approximately 3.4 million fetuses (85% of approximately 4 million live births), making it the most common obstetric procedure. From a practical viewpoint, medico-legal precedent in the US has led to a culture of electronic fetal heart rate monitoring of the majority of women in labor, despite the medical evidence that it increases the risk of operative delivery without improving perinatal outcomes in healthy, term women.
Early work going back 40 years that attempted to determine the clinical significance of fetal heart rate (FHR) patterns strongly suggested that the degree of fetal acidemia was related to depth of decelerations, whether they are late or variable decelerations. Similarly, the relationship between decreased or absent FHR variability and fetal acidemia was convincingly established, although admittedly in observational studies rather than randomized controlled trials. The 2008 National Institute of Child Health and Human Development (NICHD) document appropriately focuses on the need for further studies, particularly in Category II, where there occur most deficits in our knowledge about the risk of fetal acidemia and about how these patterns evolve into more serious patterns. Despite its widespread use, there is continued controversy about the efficacy of EFM, interobserver and intraobserver variability, nomenclature, systems for interpretation, and management algorithms. Standardization of EFM interpretation and management guidelines has been elusive, and no system is currently widely accepted in the United States.
The purpose of this document is to: 1) review nomenclature for fetal heart rate assessment, 2) review the data on the efficacy of electronic fetal monitoring (EFM), and 3) delineate the strengths and shortcomings of EFM. It also compares international three-tier systems for fetal heart rate tracing, including the NICHD, SOGC and RCOG. The collaboration of practitioners in defining the interpretation and implementing is critical for improved care for women and children. Realizing that this information deserves wide dissemination, Women's Health and Education Center (WHEC) encourages its translations and adaptations.
Background:
Intrapartum fetal assessment, a critical and ubiquitous tool in obstetrical management, is a limited and vexing part of practice. Over the last 10–15 years, progress has been made into the standardization of FHR characteristics to facilitate interpretation. The rationale behind such efforts is that guidelines formalizing the assessment of FHR patterns may improve the ability of providers to recognize and if necessary, act on EFM tracings that have been shown to correlate with poor outcomes. Whether EFM is considered a screening test or a diagnostic test is an important distinction when weighing the statistical measures of test performance: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive and negative likelihood ratios. No test performs perfectly, and typically, improvements in sensitivity or NPV lead to a reduction in specificity or PPV. Diagnostic tests usually demand strong specificity and a robust PPV, while screening tests should emphasize sensitivity and NPV. Determining what kind of test is needed can help us concentrate on the most relevant statistics. However, EFM and its associated technologies seem to combine components of both screening and diagnostic tests, depending on the evaluator's perspective. Despite the frequency of its use, limitations of EFM include poor interobserver and intraobserver reliability, uncertain efficacy, and high false-positive rate.
A complex interplay of antepartum complications, suboptimal uterine perfusion, placental dysfunction, and intrapartum events can result in adverse neonatal outcome. Known obstetric conditions, such as hypertensive disease, fetal growth restriction, and preterm birth, predispose fetuses to poor outcomes, and account for a large proportion of asphyxial injury. In a study of term pregnancies with fetal asphyxia, only 51/166 (31%) had no known risk factors (2). The fetal brain modulates the fetal heart rate through interplay of sympathetic and parasympathetic forces. Thus, FHR monitoring can be used to determine if a fetus is well oxygenated. Fetal heart monitoring may be performed externally or internally. Most external monitors use a Doppler device with computerized logic to interpret and count the Doppler signals. Internal FHR monitoring is accomplished with a fetal electrode, which is a spiral wire placed directly on the fetal scalp or other presenting part.
Fundamental Principles When Using NICHD EFM Terminology:
In April 2008, the NICHD, American College of Obstetricians and Gynecologists (ACOG), and the Society for Maternal-Fetal Medicine (SMFM) jointly sponsored a workshop on FHR patterns (3). It reevaluated the 1997 EFM recommendations, and clarified the terminology based on the ACOG document, the executive summary from the NICHD workshop, and subsequent expert articles. A set of overarching operational principles was outlined prior to presenting the actual definitions of terms integral to the interpretation of fetal monitor tracing. The most germane principles are: the definitions apply to patterns produced from either an external Doppler ultrasound device or a direct transcervical fetal electrode detecting the fetal electrocardiogram (3). The documentation of both EFM and tocodynamometry should be of adequate quality for visual interpretation. The chief emphasis is on intrapartum patterns, although the definitions are applicable to antepartum observations. The patterns defined are categorized as either baseline, periodic, or episodic. Periodic patterns are associated with contractions, whereas episodic patterns are independent of uterine contractions. Periodic patterns are distinguished based on waveform, with accelerations or decelerations defined as abrupt versus gradual onset in relation to the adjacent baseline EFM. No differentiation is made between short-term variability (or beat-to-beat variability or R-R wave period differences in electrocardiogram) and long-term variability because in practice, they are visually determined as a unit. The definition of variability is based visually on the amplitude of the complexes, with exclusion of the regular, smooth sinusoidal pattern. EFM patterns are gestational age-dependent and can differ based on fetal physiologic status, making each of these critical interpretive factors in the evaluation of an EFM pattern. Maternal medical status, prior to fetal assessments, use of medications, and other factors also warrant consideration during interpretation. The individual components of EFM that are defined do not occur in isolation and generally evolve over time. A full description of an EFM requires a qualitative and quantitative description of uterine contractions, baseline fetal heart rate, baseline variability, presence of accelerations, periodic or episodic decelerations, and changes or trends of EFM patterns over time.
To make recommendations for research priorities for EFM; a complete clinical understanding of EFM is necessary. A number of assumptions and factors common to FHR interpretation in the United States are central to the proposed system of nomenclature and interpretation. Two such assumptions are of particular importance. First, the definitions are primarily developed for visual interpretation of FHR patterns, but should be adaptable to computerized systems of interpretation. Second, the definitions should be applied to intrapartum patterns, but also are applicable to antepartum observations.
Uterine Contractions:
The number of contractions present in a 10-minute window, averaged over 30 minutes, is the manner by which uterine contractions are quantified. Contraction frequency is a partial assessment of uterine activity. Other factors such as duration, intensity, and relaxation time between contractions are equally important in clinical practice. The terminology used to describe uterine activity is listed below:
Normal: five contractions or less in 10 minutes, averaged over a 30-minute window;
Tachysystole: more than five contractions in 10 minutes, average over in a 30-minute window.
Characteristics of uterine contractions (4):
- The terms hyperstimulation and hypercontractility are not defined and should be abandoned.
- Tachysystole should always be qualified as the presence or absence of associated FHR decelerations.
- The term tachysystole applies to both spontaneous and stimulated labor. The clinical response to tachysystole may differ depending on whether contractions are spontaneous or stimulated.
Definitions of EFM Fetal Heart Rate Patterns:
The characteristics of baseline, variability, acceleration, and deceleration are essential to the definitions of FHR patterns. A normal FHR baseline rate ranges from 110 to 160 beats per minute. If the baseline FHR is less than 110 beats per minute, it is termed bradycardia. If the baseline FHR is more than 160 beats per minute, it is termed tachycardia. The following table provides EFM interpretation and descriptions based on the 2008 National Institute of Child Health and Human Development Working Group findings (3)(4). Decelerations are defined as recurrent if they occur with at least one half of the contractions.
| Pattern | Definition |
|---|---|
| Baseline |
|
| Baseline variability |
|
| Acceleration |
|
| Early deceleration |
|
| Late deceleration |
|
| Variable deceleration |
|
| Prolonged deceleration |
|
| Sinusoidal pattern |
|
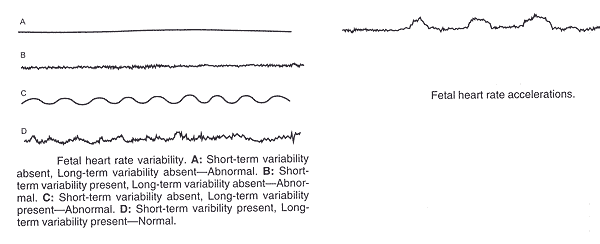
Guidelines for Review of EFM:
EFM should be reviewed during labor frequently by the nurses, physicians, or midwives. In a patient without complications, the EFM tracing should be reviewed approximately every 30 minutes in the first stage of labor and every 15 minutes during the second stage. The corresponding frequency for patients with complications (e.g., fetal growth restriction, preeclampsia) is approximately every 15 minutes in the first stage of labor and every 5 minutes during the second stage. Healthcare providers should periodically document that they have reviewed the tracing. The EFM tracing, as part of the medical record, should be labeled and available for review if the need arises. Computer storage of the FHR tracing that does not permit overwriting or revisions are reasonable, as is microfilm recording. The efficacy of EFM during labor is judged by its ability to decrease complications, such as neonatal seizures, cerebral palsy, or intrapartum fetal death, while minimizing the need for unnecessary obstetric interventions, such as operative vaginal delivery or cesarean delivery. There are no randomized clinical trials to compare the benefits of EFM with any form of monitoring during labor (5)(6). Thus the benefits of EFM are gauged from reports comparing it with intermittent auscultation.
n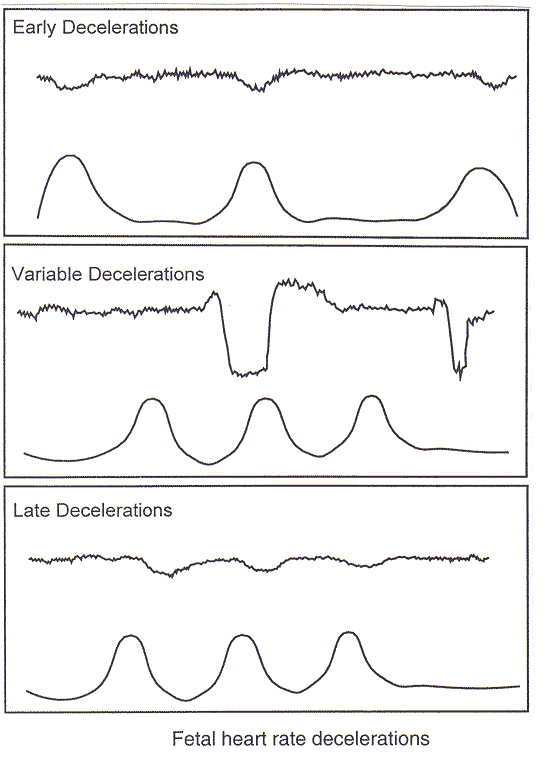
Interpretative Systems for Classification of Fetal Heart Rate Tracings:
Although many interpretative systems exist for EFM tracings, the selected system must be evidence based, simple, and applicable to clinical practice. As the FHR response is a dynamic process that requires frequent reassessment, categorization of a tracing is limited to the time period being assessed. Over time it is not uncommon for EFM tracing to migrate from one category to another. EFM tracing patterns provide information on the current acid-base status of the fetus and cannot predict the development of cerebral palsy. Two FHR findings reliably predict the absence of acidemia: 1) the presence of FHR acceleration, either spontaneous or stimulated, or 2) moderate FHR variability. It must be emphasized, however that although either fetal accelerations or moderate FHR variability reliably predict the absence of acidemia, the absence of accelerations or moderate FHR variability reliably predict the absence of acidemia, the absence of accelerations, the presence of minimal variability does not reliably predict the presence of fetal hypoxemia or metabolic acidemia (3)(7). The significance of marked variability (formerly described as salutatory) remains unclear. Although the entire associated clinical circumstances must always be taken into account, the 2008 NICHD workshop has simplified categorization and interpretation of FHR tracings into a 3-tier system.
Three-Tiered Fetal Heart Rate Interpretation System (3):
Category I
Category I FHR tracings are normal and are strongly predictive of normal acid-base status at the time of observation. Category I FHR tracings may be monitored in a routine manner and no specific action is required. It should include all of the following:
- Baseline rate: 110 – 160 beats per minute;
- Baseline FHR variability: moderate;
- Late or variable decelerations: absent;
- Early decelerations: present or absent;
- Accelerations: present or absent.
Category II
Category II FHR tracings are indeterminate. These FHR tracings are not predictive of abnormal fetal acid-base status, yet presently there is not adequate evidence to classify these as Category I or Category III. Category II FHR tracings require evaluation, taking into account the entire associated clinical circumstances. In some circumstances, either ancillary tests to ensure fetal well-being or intrauterine resuscitative measures may be used with Category II tracings. Examples of Category II FHR tracings include any of the following:
Baseline rate
- Bradycardia not accompanied by absent baseline variability;
- Tachycardia;
Baseline FHR variability
- Minimal baseline variability;
- Absent baseline variability with no recurrent decelerations;
- Marked baseline variability;
Accelerations
- Absence of induced accelerations after fetal stimulation;
Periodic or episodic decelerations
- Recurrent variable decelerations accompanied by minimal or moderate baseline variability;
- Prolonged deceleration more than 2 minutes but less than 10 minutes;
- Recurrent late decelerations with moderate baseline variability;
- Variable decelerations with other characteristics such as slow return to baseline, overshoots, or "shoulders".
Category III
Category III FHR tracings are abnormal. These are associated with abnormal fetal acid-base status at the time of observation. Category III FHR tracings require prompt evaluation. Depending on the clinical situation, efforts to expeditiously resolve the abnormal FHR pattern may include but are not limited to provision of maternal oxygen, change in maternal position, discontinuation of labor stimulation, treatment of maternal hypotension, and treatment of tachysystole with FHR changes. If a Category III tracing does not resolve with these measures, delivery should be undertaken. Category III FHR tracings include either:
- Absent baseline FHR variability and any of the following:
- Recurrent late decelerations;
- Recurrent variable decelerations;
- Bradycardia;
- Sinusoidal pattern.
Ancillary tests in the management of Category II or Category III FHR tracings:
There are some ancillary tests available that help to ensure fetal well-being in the face of a Category II or Category III FHR tracing, thereby reducing the high false-positive rate of EFM. In the case of an EFM tracing with minimal or absent variability and without spontaneous acceleration, an effort should be made to elicit one. A meta-analysis of 11 studies of intrapartum fetal stimulation noted that four techniques are available to stimulate the fetus: 1) fetal scalp sampling, 2) Allis clamp scalp stimulation, 3) vibroacoustic stimulation, and 4) digital scalp stimulation (8). Because vibroacoustic stimulation and digital scalp stimulation are less invasive than the other two methods, they are the preferred methods. When there is an acceleration following stimulation, acidemia is unlikely and labor can continue. When a Category III FHR tracing is persistent, a scalp blood sample for the determination of pH or lactate may be considered. However, the use of scalp pH assessment has decreased, and this test may not even be available at some tertiary hospitals. There are likely many reasons for this decrease, including physician experience, difficulty in obtaining and processing and adequate sample in a short amount of time, and the need for routine maintenance and calibration of laboratory equipment that may be used infrequently. More importantly, scalp stimulation, which is less invasive, provides similar information about the likelihood of fetal acidemia as does scalp pH.
There are some data to suggest that fetal scalp lactate levels have higher sensitivity and specificity than scalp pH. However, a recent large randomized clinical trial that compared the use of scalp pH assessment to scalp lactate level assessment in cases of intrapartum fetal distress did not demonstrate a difference in the rate of acidemia at birth, Apgar scores, or neonatal intensive care unit admissions (9). Although scalp stimulation has largely replaced scalp pH and scalp lactate assessment in the United States, if available, these tests may provide additional information in the setting of a Category III tracing. Pulse oximetry has not been demonstrated to be clinically useful test in evaluation fetal status (10).
Methods of intrauterine resuscitation that can be used for Category II or Category III tracings:
A Category II or Category III FHR tracing requires evaluation of the possible causes. Initial evaluation and treatment may include the following:
- Discontinuation of any labor stimulating agent;
- Cervical examination to determine umbilical cord prolapse, rapid cervical dilation, or descent of the fetal head;
- Changing maternal position to left or right lateral recumbent position, reducing compression of the vena cava and improving uteroplacental blood flow;
- Monitoring maternal blood pressure level for evidence of hypotension, especially in those with regional anesthesia (if present, treatment with volume expansion or with ephedrine or both, or phenylephrine may be warranted);
- Assessment of patient for uterine tachysystole by evaluating uterine contraction frequency and duration.
Supplemental maternal oxygen is commonly used in cases of indeterminate or abnormal pattern. There are no data on the efficacy of safety of this therapy. Often, the FHR patterns persist and do not respond to change in position or oxygenation. In such cases, the use of tocolytic agents has been suggested to stop uterine contractions and perhaps avoid umbilical cord compression. A meta-analysis reported the pooled results of three randomized clinical trials that compared tocolytic therapy (terbutaline, hexoprenaline, or magnesium sulfate) with untreated controls in the management of a suspected nonreassuring FHR tracing (12). Compared with no treatment, tocolytic therapy more commonly improved the FHR tracing. However, there were no differences in rates of perinatal mortality, low 5-minute Apgar score, or admission to the neonatal intensive care unit between the groups (possibly because of the small sample size). Thus, although tocolytic therapy appears to reduce the number of FHR abnormalities, there is insufficient evidence to recommend it. Tachysystole with associated FHR changes can be successfully treated with β2-adrenergic drugs (hexoprenaline or terbutaline). A retrospective study suggested that 98% of such cases respond to treatment with a β-agonist (12). When the FHR tracing includes recurrent variable decelerations, amnioinfusion to relieve umbilical cord compression may be considered. Amnioinfusion can be done by bolus or continuous infusion technique. A randomized trial compared the two techniques of amnioinfusion and concluded that both have a similar ability to relieve recurrent variable deceleration (11). Another common cause of a Category II or Category III FHR pattern is maternal hypotension secondary to regional anesthesia. If maternal hypotension is identified and suspected to be secondary to regional anesthesia, treatment with volume expansion or intravenous ephedrine or both is warranted.
Thick meconium staining of the amniotic fluid and abnormal fetal heart rate tracing patterns:
Although meconium staining of the amniotic fluid (MSAF) is associated with an increased risk for perinatal morbidity or mortality, the association between specific fetal tracing abnormalities and neonatal outcomes remains to be defined. In the context of MSAF, routine FHR monitoring has been recommended to screen for early signs of fetal hypoxia. Fetal heart tracing abnormalities and patterns are useful indicators of perinatal mortality and/or neonatal morbidity in the context of meconium stained amniotic fluid (13). The significance of FHR on the risk for adverse outcomes in the presence of MSAF remains controversial. This study demonstrated that the presence of severe variable decelerations, prolonged decelerations, bradycardia, and tachycardia in FHR tracing patterns was associated with an increased risk of perinatal mortality and/or neonatal morbidity (14). The qualitative indicator, the presence of markedly abnormal tracing, had the strongest association with perinatal mortality and/or neonatal morbidity. The management of such abnormalities must take into consideration several factors, including the presence of fetal tachycardia or bradycardia, duration of abnormalities, and the expected time of delivery. In the presence of thick meconium staining of the amniotic fluid, marked FHR tracing abnormalities are associated with more than twice the risk for perinatal mortality and/or neonatal morbidity relative to a normal tracing (14). Moderate FHR tracing abnormalities are associated with increased risk for perinatal mortality and/or neonatal morbidity. The clinician managing tracing abnormalities in the presence of thick meconium staining of the amniotic fluid must consider the specific tracing abnormalities (fetal tachycardia or bradycardia, type of decelerations), the duration of abnormalities, and expected time of delivery.
Comparison of Classification Systems: NICHD (U.S.A.), SOGC (Canada), RCOG (U.K.)
| NICHD (US) | SOGC (Canada) | RCOG (UK) | |
|---|---|---|---|
| Recommended intrapartum fetal surveillance for term, low-risk women | No recommendation | Intermittent auscultation | Intermittent auscultation |
| EFM Classification First tier |
Category I | Normal | Normal |
| Baseline rate: 110 - 160 beats per minute; Baseline FHR variability: moderate; |
Baseline: 110-–160 bpm Variability: 6–25 bpm -5 bpm for 40 min. Decelerations: None or occasional uncomplicated variables or early decelerations Accelerations: Spontaneous accelerations present (FHR increases - 15 bpm lasting for 15 seconds (> 32 weeks' gestation increase in the FHR 10 bpm lasting for 10 seconds) Accelerations present with fetal scalp stimulation |
An FHR trace in which all four features are classified as reassuring: Baseline: 110–160 Variability: ≥5 Decelerations: None Accelerations: Present |
|
| NICHD (US) | SOGC (Canada) | RCOG (UK) | |
| EFM Classification Second tier |
Category II | Atypical | Suspicious |
| Baseline rate Bradycardia not accompanied by absent baseline variability; Tachycardia; Baseline FHR variability Minimal baseline variability; Absent baseline variability with no recurrent decelerations; Marked baseline variability; Accelerations Absence of induced accelerations after fetal stimulation; Periodic or episodic decelerations Recurrent variable decelerations accompanied by minimal or moderate baseline variability; Prolonged deceleration more than 2 minutes but less than 10 minutes; Recurrent late decelerations with moderate baseline variability; Variable decelerations with other characteristics such as slow return to baseline, overshoots, or "shoulders". |
Baseline: Bradycardia: 100–110 bpm Tachycardia: 160 for 30 min to < 80 min. Rising baseline Variability: 5 bpm for 40–80 min. Decelerations: Repetitive - 3 uncomplicated variable decelerations Occasional late decelerations Single prolonged deceleration for 2 min. but <3 min. Accelerations: Absence of acceleration with fetal scalp stimulation |
An FHR trace with one feature classified as non-reassuring and the remaining features classified as reassuring: Baseline: 100–109 bpm 161–180 bpm Variability: < 5 for 40–90 minutes Decelerations: Typical variable decelerations with over 50% of contractions, occurring for over 90 minutes Single prolonged deceleration for up to 3 minutes Accelerations: The absence of accelerations with otherwise normal trace is of uncertain significance |
|
| NICHD (US) | SOGC (Canada) | RCOG (UK) | |
| EFM Classification Third Tier |
Category III | Abnormal | Pathological |
| Absent baseline FHR variability and any of the following: Recurrent late decelerations; Recurrent variable decelerations; Bradycardia; •Sinusoidal pattern. |
Baseline: Bradycardia:100 bpm Tachycardia: 160 for80 min. |
An FHR trace with two or more features classified as non-reassuring or one or more classified as abnormal: Baseline: < 100 bpm > 180 bpm Sinusoidal pattern • 10 minutes Variability: < 5 for 90 minutes Decelerations: Either atypical variable decelerations with over 50% of contractions or late decelerations, both for over 30 minutes Single prolonged deceleration for more than 3 minutes Accelerations: Left "blank" in document |
Future Possibilities:
Classic EFM, appropriate for both high- and low-risk patients, uses intervals between heart beats (for external or Doppler monitoring) or between R waves (for internal or electrocardiogram – ECG – monitoring) to calculate heart rates. This calculated rate is then mapped to the FHR strip to demonstrate characteristics that can be read by a provider. As a result, the technology is dependent on the knowledge and experience of the interpreter. Recent technological advances, such as the STAN S31 ECG FHR monitor (Neoventa Medical AB, M怀lndal, Sweden), show promise in the monitoring of higher-risk patients. An FDA-approved device, the STAN uses internal fetal monitoring to examine specific ECG changes, such as T-wave amplitude and ST-interval duration; much like an adult ECG. The device alerts the provider to events associated with fetal hypoxemia and/or acidemia. Study results indicate that STAN might reduce the incidence of fetal scalp pH sampling (relative risk [RR], 0.65; 95% confidence interval [CI], 0.59-0.72) and operative vaginal delivery (RR, 0.88; 95% CI, 0.80-0.97) (15). In addition to incorporating STAN ECG readings and alerts, the Omniview-Sis-Porto 3.5 (Speculum, Lisbon, Portugal) warns of FHR changes associated with fetal hypoxemia or acidemia; examples are reduced variability or tachycardia. One aim is to reduce human error in interpretation of tracings and possible delays in taking appropriate action. Another goal is to supplement the technology already in use – traditional EFM and STAN ECG – to improve the specificity of the tests. Small exploratory studies of promising new technologies need to be followed by larger clinical trials. Fetal oximetry is a good example of an intrapartum test that was appropriately analyzed with large, rigorous trials that demonstrated little additional benefit overall.
Because both the STAN ECG and Omniview-Sis-Porto 3.5 are reserved for high-risk patients and require internal fetal monitoring, neither will replace standard external EFM. Improvements in EFM interpretation may be seen with the introduction of the new category system outlined in the 2008 National Institute of Child Health and Human Development workshop report, but the clinical application of these recommendations has just begun, and no studies exist at present to demonstrate their validity or utility. Evaluation and refinement of these recommendations may be our best effort at remedying the problematic application of EFM in our daily practice.
Summary:
For practical and medical-legal reasons, obstetricians in the United States have accepted EFM as a standard component of labor management. Standardization of terminology and subsequent categorization into three-tiers should aid healthcare providers who are deciding whether the patterns are suggestive of a lack of fetal acidemia or alternately require intervention. Given that we have accepted this technology, we can improve the consistency of our approach to interpreting EFM patterns by adopting a uniform set of definitions of what is normal and what is abnormal. Focusing on FHR variability and correctly identifying the type of deceleration that is present are the two best ways to achieve a unified approach to using FHR patterns to guide management of labor. Despite compelling evidence demonstrating no neonatal benefit, the medico-legal climate in the United States requires obstetricians to integrate continuous intrapartum surveillance into their care of the pregnant patient. In Canada, the appropriate use of intermittent auscultation during labor has been upheld in the court of law, but as with EFM, the procedure and interpretation must be conducted according to professional practice standards. The false-positive rate of EFM for predicting cerebral palsy is high, at greater than 99%. The use of EFM is associated with an increased rate of both vacuum and forceps operative vaginal delivery, and cesarean delivery for abnormal FHR patterns or acidosis or both. When the EFM tracing includes recurrent variable decelerations, amnioinfusion to relieve umbilical cord compression should be considered. Pulse oximetry has not been demonstrated to be a clinically useful test in evaluating fetal status.
There is high interobserver and intraobserver variability in interpretation of FHR tracing. Reinterpretation of the FHR tracing, especially if the neonatal outcome is known, may not be reliable. The use of EFM does not result in a reduction of cerebral palsy. A three-tiered system for the categorization of FHR patterns is recommended. The labor of women with high-risk conditions should be monitored with continuous FHR monitoring. The terms hyperstimulation and hypercontractility should be abandoned. Personal review of the original NICHD document is strongly encouraged.
Acknowledgment:
Special thanks to Dr. Peter von Dadelszen, MBCHB, DPhil, Associate Professor of Obstetrics and Gynecology (Maternal-Fetal Medicine) at the University of British Columbia (UBC) and Consultant in Maternal-Fetal Medicine, Children's and Women's Health Centre of British Columbia (CWHCBC), University of British Columbia, Department of Obstetrics and Gynecology, and Dr. Diane Sawchuck, RN, PhD, Knowledge Translation Scientist, Reproduction & Healthy Pregnancy Cluster, Child & Family Research Institute, Co-Investigator, Optimal Birth BC School of Population and Public Health, Adjunct Professor, School of Nursing, University of British Columbia, Vancouver, Canada for reviewing the manuscript and expert opinions.
References:
- Martin JA, Hamilton BE, Sutton PD et al. Births: final data for 2002. Natl Vital Stat Rep 2003;52:1-113. (Level II-3)
- Low JA, Pickersgill H, Killen H et al. The prediction and prevention of Intrapartum fetal asphyxia in term pregnancies. Am J Obstet Gynecol 2001;184:724-730
- Macones GA, Hankins GDV, Spong CY et al. The 2008 National Institute of Child Health and Human Development Workshop Report on Electronic Fetal Monitoring Interpretation, and Research Guidelines. Obstet Gynecol 2008;112:661-666
- American College of Obstetricians and Gynecologists Practice Bulletin. Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Number 106, July 2009. Obstet Gynecol 2009; 114:192-202
- Parer JT, Ikeda R. A framework for standardized management of Intrapartum fetal heart rate patterns. Am J Obstet Gynecol 2007;197:26.e1-6
- Freeman RK. Problems with intrapartum fetal heart rate monitoring interpretation and patient management. Obstet Gynecol 2002;100:813-826 (Level III)
- Robinson B. A review of NICHD standardized nomenclature for cardiotocography: the importance of speaking a common language when describing electronic fetal heart monitoring. Rev Obstet Gynecol 2008;1:56-60
- Skupski DW, Rosenberg CR, Eglinton GS. Intrapartum fetal stimulation tests: a meta-analysis. ObstetGynecol 2002;99:129-134. (Meta-analysis)
- Wiberg-Itzel E, Lipponer C, Norman M et al. Determination of pH or lactate in fetal scalp blood in management of intrapartum fetal distress: randomized controlled multicentre trial. BMJ 2008;336:1284-1287. (Level I)
- Bloom SL, Spong CY, Thom E et al. Fetal pulse oximetry and cesarean delivery. National Institute of Child Health and Human Development Maternal-Fetal Unites Network. N Engl J Med 2006;355:2195-2202. (Level I)
- Rinehart BK, Terrone DA, Barrow JH et al. Randomized trial of intermittent or continuous amnioinfusion for variable decelerations. Obstet Gynecol 2000;96:571-574. (Level I)
- ACOG Practice Bulletin. Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Number 106, July 2009. Obstet Gynecol 2009;114:192-202
- Parer JT, King T, Flanders S et al. Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association? J Matern Fetal Neonatal Med 2006;19:289-294
- Xu H, Mas-Calvet M, Wei S-Q et al. Abnormal fetal heart rate tracing patterns in patients with thick meconium staining of the amniotic fluid: association with perinatal outcomes. Am J Obstet Gynecol 2009;200:283.e1-283.e7
- Costa A, Ayres-de-Campos D, Costa F et al. Prediction of neonatal acidemia by computer analysis of fetal heart rate and ST event signals. Am J Obstet Gynecol 2009;201:464.e1-6
Published: 11 December 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


