Stillbirth: Evaluation and Management
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Stillbirth is one of the most devastating, as well as common, obstetric complications, affecting over 3 million pregnancies per year throughout the world (1). A major unanswered question is the optimal diagnostic evaluation for cases of stillbirth. Important psychologic and emotional issues arise when dealing with a pregnancy resulting in a stillbirth. Couples often experience feelings of anxiety, failure, personal guilt, and apprehension when contemplating pregnancy after having a stillborn infant. Many women do not receive comprehensive counseling regarding the cause of the stillbirth because an incomplete evaluation was performed, and in 50% of cases the cause remains unknown. It is difficult for clinicians to counsel, evaluate, and manage subsequent pregnancies optimally because very little is known about pregnancy outcome after a stillbirth. There are several reasons why it can be difficult to ascertain a “cause” of stillbirth. First, the cause of stillbirth may be complex and multifactorial. Several conditions simultaneously occurring may contribute to a given stillbirth and it may not be possible to determine a single proximate cause. Second, many conditions are “risk factors” rather than causes of stillbirth. These conditions are often present in pregnancies with live births. Examples include smoking, obesity, or well-controlled diabetes. Third, a cause of death may not be identified, even after a thorough evaluation of stillbirth. Finally, there are likely causes of stillbirth that have yet to be discovered.
The purpose of this document is to review the current information on stillbirth, including definitions and management, the evaluation of a stillbirth, and strategies for prevention. There is a paucity of information on the outcome of pregnancies after stillbirth. Prior stillbirth is associated with a 2-fold to 10-fold increased risk of stillbirth in the future pregnancy. The risk depends on the etiology of the prior stillbirth, presence of fetal growth restriction, gestational age of the prior stillbirth, and race. The term stillbirth is preferred among parent groups, and more recent research efforts have begun using this term in place of fetal death. Therefore, in this document, the term stillbirth is used. It must be emphasized that the criteria for stillbirth do not imply a point of viability and were chosen to facilitate uniform data collection. In the United States, fetal losses related to termination of pregnancy for lethal fetal anomalies and inductions of labor for previable premature rupture of membranes are specifically excluded from the stillbirth statistics and are classified as termination of pregnancy.
Epidemiology of Stillbirth
Stillbirth is one of the most common adverse pregnancy outcomes in the United States, occurring in one out of every 200 pregnancies, which amounts to approximately 26,000 stillbirths every year (2). The stillbirth rate in the United States has decreased slightly over the past 15 years, with a rate of 6.2 per 1,000 live births in 2003 (2). This is almost 1 in 160 pregnancies. The decrease in stillbirths has primarily been in stillbirths after 28 weeks’ gestation. Many stillbirths are from genetic causes. These include aneuploidy, syndromes, malformations, and single-gene (including Mendelian) disorders. Stillbirths constitute half of all perinatal mortality, and 50% have an undetermined cause of death. There is significant racial disparity in the stillbirth rate; the rate for non-Hispanic black women is more than double that of non-Hispanic white women (2).
The rate of stillbirth has decreased substantially from the 1950s (20 per 1,000 births) through the 1980s with improved care for conditions such as diabetes, red cell alloimmunization, and preeclampsia. However, stillbirth rates have been relatively stable over the past 20 years, reaching a plateau in the United States. Since 1990, the rate of early stillbirth (20-27weeks), has remained stable at approximately 3.2 per 1,000 births, while the rate of late stillbirths (28 weeks or greater) has decreased from 4.3 to 3.1 per 1,000 births. In 2004, the stillbirth rate in the United States was 6.2 per 1,000 down from 6.4 per 1,000 births in 2002 (2). In developed countries, the most prevalent risk factors associated with stillbirth are non-Hispanic black race, nulliparity, advanced maternal age, and obesity. From a public health perspective, obesity, smoking, and drug and alcohol use are common potentially modifiable risk factors for adverse pregnancy outcome. In 2001, African Americans suffered a stillbirth rate of 12.1 per 1,000 births compared with 5.5 per 1,000 for whites (3).
Definition
Definitions of stillbirth (and thus stillbirth rates) vary in different countries, based on gestational age. In the United States, stillbirth (defined as fetal death at >20 weeks’ gestation) affects about 1 in 160 pregnancies (6-7 per 1,000 births). The rate is similar in most high-income countries (3-5 per 1,000 births), but is considerably higher (20-100 per 1,000 births) in low- and middle-income countries (1). The United States National Center for Health Statistics defines fetal death (stillbirth) as the delivery of a fetus showing no signs of life as indicated by the absence of breathing, heart beats, pulsation of the umbilical cord, or definite movements of voluntary muscles (4). There is not complete uniformity among states with regard to birth weight and gestational age criteria for reporting fetal deaths. However, the suggested requirement is to report fetal death at 20 weeks or greater of gestation (if the gestational age is known), or a weight greater than or equal to 350 grams if the gestational age is not known. The cutoff of 350 grams is the 50th percentile for weight at 20 weeks of gestation.
Classification of Stillbirth
In many cases it is difficult to be certain of the etiology of stillbirth. First, many cases are unexplained, despite intensive investigation of potential causes. Second, more than one condition may contribute to stillbirth in an individual case. For example, infection could occur in a fetus with trisomy 18. It may not be possible to precisely determine which disorder was directly responsible for the loss. Indeed, it is likely that some cases of stillbirth are due to contributions from multiple factors. Finally, conditions may be associated with stillbirth without directly causing them. These concerns have led to the development of several classification systems for causes of stillbirth. No single classification system is universally accepted, and each has strengths and weaknesses. In 1980 Wigglesworth and collaborators introduced the 9 category classification system that is currently most commonly used in reporting perinatal mortality rates (5)
I. Wigglesworth Classification:
- Congenital defect/malformation (lethal or severe);
- Unexplained antepartum fetal death;
- Death from intrapartum asphyxia, anoxia, or trauma;
- Immaturity;
- Infection;
- Death due to other specific causes;
- Sudden infant death, cause unknown;
- Unclassifiable.
Gardosi et al recently proposed a new classification scheme in which neonatal deaths are excluded (6). Although this classification mentions the coding of primary and secondary “causes” of stillbirth, the goal is to identify the relevant conditions present at the time of death in utero. It is a hierarchical classification in which the hierarchy starts from the conditions directly affecting the fetus and moves outward in anatomical groups. The authors demonstrated a significant decrease of unclassified stillbirth when compared with Wigglesworth’s classification. These authors emphasize the important contribution of fetal growth restriction, with approximately 50% of stillbirth associated with fetal growth restriction
II. ReCoDe (relevant condition of death) classification:
- Fetus: lethal congenital anomaly; infection, chronic (e.g., TORCH*), acute; non-immune hydrops; isoimmunization; feto-maternal hemorrhage; twin-twin transfusion; intrapartum asphyxia; fetal growth restriction; other.
- Umbilical Cord: prolapse; constricting loop or knot; velamentous insertion; other.
- Placenta: abruption; previa; vasa previa; placental infarction; other placental insufficiency; other.
- Amniotic fluid: chorioamnionitis; oligohydramnios; polyhydramnios; other.
- Uterus: rupture; uterine anomalies; other.
- Mother: diabetes; thyroid disease; essential hypertension; hypertensive diseases in pregnancy; lupus/antiphospholipid syndrome; cholestasis; drug abuse; other.
- Trauma: external; iatrogenic.
- Unclassified: no relevant condition identified; no information available.
*TORCH: toxoplasmosis, other infections, rubella, cytomegalovirus, and herpes simplex virus
Risk Factors
Consistent demographic factors for stillbirth include race, low socioeconomic status, inadequate prenatal care, less education, and advanced maternal age (7). African-American women have rates of stillbirth that are more than twice the rate for white mothers. In part, this may be due to secondary risk factors such as socioeconomic status and a lack of prenatal care
Commonly Reported Maternal Risk Factors and Causes for Stillbirth (8):
| Developed Countries | Developing Countries |
| Congenital and karyotypic anomalies; | Obstructed and prolonged labor and associated asphyxia, infection, and birth injury; |
| Growth restriction and placental abnormalities; | Infection especially syphilis and gram-negative infections; |
| Medical diseases such as diabetes, systemic lupus erythematous, renal disease, thyroid disease, and cholestasis of pregnancy; | Hypertensive disease and complications of preeclampsia and eclampsia; |
| Hypertensive disease and preeclampsia; | Congenital anomalies; |
| Infection such as human parvovirus 19, syphilis, streptococcal infection, and listeria; | Poor nutritional status; |
| Smoking; | Malaria; |
| Multiple gestation; | Sickle cell disease; |
Chromosomal and Genetic Abnormalities
An abnormal karyotype can be found in approximately 8-13% of stillbirths (9). The rate of karyotypic abnormalities exceeds 20% in fetuses with anatomic abnormalities or in those with growth restriction, but the rate of chromosomal anomalies found in normally formed fetuses was found to be 4.6% in one large series (9). If an abnormal karyotype is found in association with stillbirth, the most common abnormalities are monosomy X (23%), trisomy 21(23%), trisomy 18(21%), and trisomy 13(8%). Confined placental mosaicism also has been associated with an increased risk of stillbirth, but currently is not part of standard testing (10). Karyotypic analysis underestimates the contribution of genetic abnormalities to stillbirth because in up to 50% of karyotype attempts cell culture is unsuccessful (10). One strategy to increase the yield of cell culture is to perform an amniocentesis before the delivery. This is typically performed after the woman has had an opportunity to process the death of her baby and after an epidural is placed. In a large Dutch study, invasive testing has a much greater tissue culture rate (85%) than fetal tissue sampling after birth (28%). In addition, routine assessments for single gene defects and microdeletions currently are not recommended because of uncertainty of the role of these genetic anomalies. However, it is likely that no single-gene defect is likely to be responsible for a significant proportion of stillbirth. Genetic evaluation for specific abnormalities should be guided by the clinical history and detected fetal abnormalities. Approximately 20% of stillborn fetuses have dysmorphic features or skeletal abnormalities and 15-20% has a major malformation (11).
Congenital Malformations
Although some have recommended that extensive postnatal evaluation of the fetus be performed only in selected cases, the frequent occurrence of unexpected and unanticipated findings is argument for routine, comprehensive assessment of all stillbirths (12). Using such an approach, around 20% of stillborns will have detectable congenital anomalies as fetal causes of death. A malformation can be considered the cause of death based on specific criteria; fulfilling any one of the following is sufficient to postulate that a particular process is causal:
- There are epidemiologic data demonstrating an excess of intrauterine mortality (e.g., Turner syndrome, Down syndrome, trisomy 18, and Smith Lemli Optiz Syndrome).
- The process is seen rarely in live-born neonates (e.g., lower mesodermal defects, triploidy, and early amnion disruption).
- When the process is seen in live-born neonates, it frequently results in neonatal death (anencephaly, congenital cardiomyopathy, hydranencephaly, and limb-body wall disruption sequence).
- There is biologic plausibility that it can result in death (aprosencephaly syndrome, Meckel syndrome, and jugulolymphatic obstruction sequence).
If multiple criteria are met, the likelihood that the process caused death is increased.
Maternal Age Older Than 35 years
Older maternal age is associated with an increased risk of stillbirth in both nulliparous and multiparous women (13). A significant proportion of perinatal deaths seen in older women are related to lethal congenital and chromosomal anomalies. The introduction of population-based screening for chromosomal abnormalities has contributed to lower rates of this type of perinatal demise. Large-scale studies suggest that an increased risk of unexplained stillbirth late in pregnancy persists in older women, even after controlling for risk factors such as hypertension, diabetes, placenta previa, and multiple gestations (13), (14). In addition, there appears to be an interaction between first birth and advanced maternal age that places primiprous older women at an increased risk (13). Based on one study, the estimated risk of stillbirth is 1 in 116 in a 40-year-old nulliparous woman after 37 weeks of gestation, compared with 1 in 304 in a multiparous woman of the same age (13).
Infection
Infection has been associated with 10% to 25% of stillbirths in developed countries (15). The percentage is considerably higher in developing countries. Infection may lead to stillbirth in a variety of ways. These include direct fetal infection with damage to a vital organ, fetal deformation, placental infection leading to impaired placental function, severe maternal systemic infection leading to sepsis, and infection leading to preterm labor with intrapartum fetal death. It can be difficult to determine whether stillbirth is the result of infection because some organisms are present in healthy women who have normal pregnancies. Caution is advised before assuming that infection is a cause of stillbirth.
Bacterial infections: numerous bacteria have been implicated as causes of stillbirth. Many of these are vaginal flora that reaches the upper genital tract through the cervix. They may infect the deciduas and chorion, eventually reaching the fetus through the amniotic fluid. These organisms include Escherichia coli, Klebsiella, group B Streptococcus, Mycoplasma hominus, Ureaplasma urealyticum, and Bacteroides species. Rarely, bacteria such as Listeria monocytogenes can reach the fetus by hematogenous transmission. Some of these organisms may cause clinically apparent intra-amniotic infection with fever, abdominal pain, and uterine contractions. However, symptoms may be vague, making diagnosis difficult, especially in cases of infection with relatively indolent organisms such as Mycoplasmas, Ureaplasmas, and Listeria.
Viral infections: the most common viral infection is cytomegalovirus (CMV). Fetal infection is usually associated with primary maternal infection, which occurs in about 1% of pregnancies in the United States (16). Although many fetuses infected with CMV suffer adverse effects, stillbirth is rare. Because fetal CMV infection is common, and most infants survive, histologic evidence of CMV in the fetus and placenta should be present if stillbirth is to be attributed to CMV. Parvovirus B19 is most commonly linked to pregnancy loss. The virus attacks erythrocyte precursors and myocardial cells, leading to anemia, myocardial dysfunction, hydrops and in severe cases, stillbirth. Parvovirus B19 has been reported to be present in 7.5% of fetal deaths in a Swedish study that used the polymerase chain reaction to assess infection (17). Numerous other viruses have been associated with sporadic stillbirths. Coxsackie A and B viruses can cause placental inflammation, myocarditis, hydrops, and death. Other viruses include adenoviruses, echoviruses, enteroviruses, varicella, rubeola (measles), mumps, and rubella. The risk of stillbirth in association with some of these viruses can be vastly reduced with vaccination. The human immunodeficiency virus (HIV) may infect the fetus in utero, but rarely causes stillbirth. In contrast, the herpes simplex virus rarely infects, but can cause stillbirth.
Other infections: there are several common infectious causes of stillbirth, including spirochetes, parasites, and other types of organism. Syphilis, caused by Treponema pallidum, is associated with stillbirth. The spirochete may cross the placenta in the latter part of pregnancy. Stillbirth may occur either because of direct fetal infection or placental inflammation and vasculopathy in response to placental infection. The risk of stillbirth because of syphilis increases with advancing gestational age. Treponema pallidum still causes occasional stillbirths in the United States, with a congenital syphilis rate of about 10 per 100,000 (18). It is considerably more common in some endemic areas, such as the Mississippi Delta, disadvantaged urban areas, and the US-Mexico borderlands, as well as in developing countries with a higher prevalence of the conditions. Other spirochetes such as Borrelia burgdorferi, the causative agent in Lyme disease, produce sporadic stillbirths. Toxoplasma gondii is a parasite transmitted through undercooked meat or through cat feces. It may cross the placenta after maternal infection, leading to fetal infection and death. Fetal infection occurs in about 40% of cases and is more likely later in gestation (19). However, the consequences of fetal infection are more severe earlier in pregnancy. It is estimated that 5% of fetal infections between 10 and 24 weeks’ gestation result in serious problems, including death (19). Toxoplasma gondii is infrequent in the United States (about 1 per 1,000), and it is likely a rare cause of stillbirth (15). The prevalence is considerably higher in other countries.
Systemic infections: severe maternal systemic infection also can lead to stillbirth. Most systemic infections are well tolerated by the fetus. However, sepsis can lead to uterine ischemia and decreased placental perfusion, resulting in antepartum stillbirth. Alternatively, systemic infection sometimes initiates preterm labor and delivery. Especially in the setting of a previable infant, this may result in intrapartum stillbirth.
Evaluation of a Stillbirth
The most important tests in the evaluation of a stillbirth are fetal autopsy; examination of the placenta, cord, and membranes; and karyotype evaluation. In addition to the identification of birth defects and morphologic abnormalities suggesting genetic or developmental abnormalities, autopsy can determine and/or confirm numerous other causes of stillbirth. It is helpful to ask families about their specific concerns and reasons for refusing autopsy. In many instances, their fears can be alleviated with education or modification of the procedure. In cases wherein autopsy is refused, partial autopsy or postpartum magnetic resonance imaging (MRI) may provide substantial information (20). When a full autopsy is performed, it should follow published guidelines for perinatal autopsy. The pathologist should be aware of the clinical history and suspected genetic diagnoses, as well as any necessary tissue collection that needs to be performed for additional analyses. An algorithm for evaluation is given below (11).
Flow chart for fetal and placental evaluation (11):
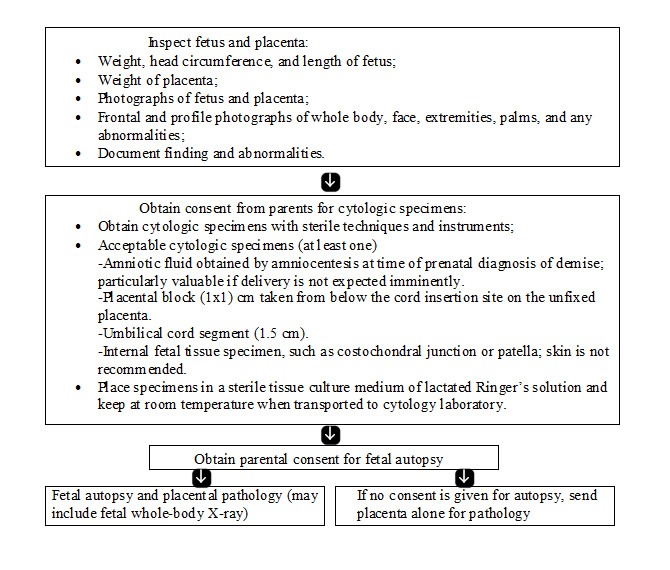
Alternatives to a Complete Autopsy
Gross and microscopic examination of the placenta is an essential component of the evaluation of any stillbirth and should include an examination of the membranes and umbilical cord that may corroborate autopsy findings. Even if the family declines fetal autopsy, histologic study of the placenta usually is acceptable and can be valuable in identifying underlying etiologies.
- Placental examination and external examination by perinatal pathologist (generally includes measurements of the baby, X-rays, and photographs). It will be more likely to identify syndromes, congenital abnormality, and timing of death as well as growth abnormalities. It will be able to detect placental and cord infections. It can miss fetal infections and internal congenital and central nervous system (CNS) anomalies.
- Placental examination and external examination by a perinatal pathologist, and selected biopsies (this generally includes measurements of the baby, X-rays, and photographs). It may be more likely to identify fetal infections; its limitations are stated above.
- Gross and microscopic placental examination and external and internal examination of the fetus by a perinatal pathologist, organs, are left with the body, and the brain is not examined (this generally includes measurements of the baby, X-rays, and photographs). It allows the baby to be returned to the family with all the organs. It will miss CNS pathology, but will detect internal congenital abnormalities and be able to assess the role of infection.
- Head sparing autopsy. It has benefits of full autopsy; may miss some CNS pathology.
- Magnetic resonance imaging (MRI) with plus or minus directed needle biopsy. It may be very useful with the family requires burial intact in a timely manner. MRI is good in the identification of CNS pathology, but other abnormalities such as cardiac abnormalities are more likely to be missed. Infections will not be diagnosed unless additional needle biopsies are considered. This strategy has not been compared with traditional autopsy.
- Ultrasound. It is best done while in utero, but can be done after birth. The head, kidney, or abdomen can be evaluated, but only static images of the heart can be seen. It is not as good as MRI for the brain, but may be able to provide some useful information if a previous fetal radiologic survey was not performed. It is limited by the degree of maceration, and does not assess role of infection in the fetal death.
Fetal Laboratory Studies
Fetal karyotype is valuable, especially in cases wherein autopsy is not performed. Chromosomal abnormalities are present in at least 6 to 12% of stillbirths (21). The risk of an abnormal karyotype is higher for fetuses with structural abnormalities, dysmorphic features or those dying earlier in gestation. These risk factors may be used to stratify risk if cost is an issue. Tissues most likely to provide cells that can successfully be grown in culture include placenta (especially chorionic plate), fascia lata, skin from the nape of the neck, and tendons. Ideally, a trained pathologist should send tissues for karyotype after gross evaluation of the fetus and placenta. If this is not possible, clinicians should take care not to place placental or fetal tissues in formalin so that attempts can be made to culture cells. Comparative genomic hybridization shows tremendous promise for the identification of chromosomal abnormalities in stillbirths wherein fetal cells cannot be successfully cultured (22). In cases of unsuccessful cell culture, clinicians should investigate whether comparative genomic hybridization is available in their center. Genetic abnormalities not identified by karyotype almost assuredly contribute to some cases of stillbirth. Additional genetic testing for specific conditions may be appropriate based on suspicion raised through perinatal autopsy. However, widespread testing for single gene disorders, microdeletions, and confined placental mosaicism is still considered experimental and the evidence to offer these tests on a routine basis is lacking. Fetal karyotype also is important if a parent carries a balanced chromosomal rearrangement (e.g. translocation or inversion) or has a mosaic karyotype. Samples of amniotic fluid, umbilical cord, fetal tissue, or placenta may be obtained for chromosomal and any other relevant tests. Amniocentesis for fetal karyotype has the highest yield and is particularly valuable if delivery is not expected imminently (9).
Maternal Evaluation
A thorough maternal history should be taken looking for known conditions or symptoms suggestive of those that have been associated with stillbirth. The key components and elements of the stillbirth history and evaluation are:
- Family history: recurrent spontaneous abortions; venous thromboembolism or pulmonary embolism; congenital anomaly or abnormal karyotype; hereditary condition or syndrome; developmental delays and consanguinity.
- Maternal history: prior venous thromboembolism or pulmonary embolism; diabetes mellitus; chronic hypertension; thrombophilia; systemic lupus erythematosus; autoimmune disease; epilepsy; severe anemia; heart disease; tobacco, alcohol, drug or medication abuse.
- Obstetric history: recurrent miscarriages; previous child with anomaly, hereditary condition, or growth restriction; previous gestational hypertension or preeclampsia; previous gestational diabetes mellitus; previous placental abruption; previous fetal demise.
- Current pregnancy: maternal age; gestational age at fetal death; medical conditions complicating pregnancy (e.g. hypertension, diabetes mellitus, systemic lupus erythematosus, cholestasis); pregnancy weight gain and body mass index; complication of multifetal gestation, such as twin-twin transfusion syndrome, twin reversed arterial perfusion syndrome, and discordant growth; placental abruption; abdominal trauma; preterm labor or rupture of membranes; gestational age at onset of prenatal care; abnormalities seen on an ultrasound image; infections or chorioamnionitis.
- Maternal evaluation at time of demise: complete blood count; fetal-maternal hemorrhage screen: Kliehauer-Betke test or comparable test for fetal cells in maternal circulation; human parvovirus B19 immunoglobulin G and immunoglobulin M antibody; syphilis; lupus anticoagulant; anticardiolipin antibodies; thyroid-stimulating hormone; thrombophilia (in selected cases) – factor V Leiden, prothrombin gene mutation, antithrombin III, fasting homocysteine.
- Postpartum: protein S and protein C activity (in selected cases); parental karyotype (if appropriate); Indirect Coombs test; glucose screening (oral glucose tolerance test, hemoglobin A1C); toxicology screen. In selected cases antinuclear antibody test and TORCH serology. Rarely helpful, infection causing death is made by history and examining the baby, placenta, and cord.
- Developing technologies (22): comparative genomic hybridization; testing for single-gene mutations; testing for confined placental mosaicism and nucleic acid-based testing for infection.
Management of Current Pregnancy with Fetal Death
Most women prefer to proceed with delivery of the fetus after diagnosis of fetal death. It is emotionally stressful to carry a nonviable fetus, especially late in gestation. It is important to offer both the options of delivery and expectant management to women experiencing fetal death. Risks of expectant management include intrauterine infection and maternal coagulopathy. These risks are poorly characterized due to the relative infrequency of expectant management. Older reports state that 80-90% of women will spontaneously labor within two weeks of fetal death (3). However, this “latency” period may be substantially longer. It seems prudent to perform surveillance for infection and coagulopathy in women undergoing expectant care. Examples include serial assessment of maternal temperature, abdominal pain, bleeding, and labor. Regular office visits (e.g. weekly) may be useful for emotional support and medical surveillance. Some authorities advise serial (e.g. weekly) determination of complete blood count, platelet count, and fibrinogen level. The usefulness of this is uncertain. A consumptive coagulopathy has been reported in 25% of patients who retain a dead fetus for more than 4 weeks (23), but the condition is rare in clinical practice and is not usually associated with clinical sequelae. A fibrinogen level of less than 100mg/dL is considered evidence of coagulopathy. Patients should be advised to immediately report symptoms such as fever, pain, bleeding, contractions, leaking fluid, or foul discharge.
The method and timing of delivery after a fetal death depends on the gestational age at which the death occurred, on the maternal history of a previous uterine scar, and maternal preference. Although most patients will desire prompt delivery, the timing of delivery is not critical; coagulopathies are associated with prolonged fetal retention are uncommon. In the second trimester, dilatation and evacuation can be offered if an experienced provider is available, although patients should be counseled that dilatation and evacuation may limit efficacy of autopsy for the detection of macroscopic fetal abnormalities. Labor induction is appropriate at later gestational ages. Before 28 weeks of gestation, vaginal misoprostol appears to be the most efficient method of induction, regardless of cervical Bishop score (24), although high-dose oxytocin infusion also is an acceptable choice. Typical dosages for misoprostol use are 200-400 mcg vaginally every 4-12 hours. After 28 weeks of gestation, induction of labor should be managed according to usual obstetric protocols. Cesarean delivery for fetal demise should be reserved for unusual circumstances because it is associated with potential maternal morbidity without any fetal benefit. Several studies have evaluated the use of misoprostol at a dosage of 400 mcg every 6 hours in women with a stillbirth between 24 and 28 weeks of gestation and a prior uterine scar (25). Available evidence from randomized trials supports the use of vaginal misoprostol as a medical treatment to terminate non-viable pregnancies before 24 weeks of gestation (25). Further research is required to assess effectiveness and safety, optimal route of administration, and dose. In patients after 28 weeks of gestation, cervical ripening with a transcervical Foley catheter has been associated with uterine rupture rates comparable to spontaneous labor and this may be helpful adjunct in patients with an unfavorable cervical examination (26). Therefore, based on limited data in patients with a prior low transverse cesarean delivery, trial of labor remains a favorable option. There are limited data to guide clinical practice in a patient with prior classical cesarean delivery, and the delivery plan should be individualized.
The risk of stillbirth after 32 weeks of gestation increases with gestational age, and half of these late fetal deaths occur at term, especially in older women more than 35 years of age (13). Term stillbirth theoretically can be avoided through the judicious use of labor induction, and stillbirth prevention lies at the heart of many of the accepted indications for labor induction. However, once the child is born, he or she faces new mortality risks, often risks that may be determined partially by gestational age at birth. Determining the optimal time of delivery to minimize the risk of stillbirth necessarily must include considering the mortality risk faced by the child after birth. For non-anomalous infants born at term, the most common causes of death are asphyxia, infection, and sudden infant death syndrome (SIDS). Rates of infection and SIDS decrease with increasing gestational age at term, with the highest rates at 37 weeks (31). The risk of both neonatal and infant death has been shown in multiple studies to decrease with gestational age at term but then increase again at 41 weeks of gestation (31), (32). Part of relationship between gestational age and infant death is driven by the fact that SIDS is the leading cause of post-neonatal death in non-anomalous infants (33). This recent study (34) estimates the multiple dimensions of risk faced by pregnant women and their healthcare providers when comparing the risks of stillbirth at term with the risk of infant death after delivery. The conclusion was, infant mortality rates at 39, 40, and 41 weeks of gestation are lower than the overall mortality risk of expectant management for 1 week. The current study suggests that delivery carries a greater mortality risk than expectant management at 37 weeks of gestation, carries equivalent risk at 38 weeks of gestation, but becomes advantageous at 39 weeks of gestation and beyond.
Bereavement
The facilitation of bereavement is an important opportunity for clinicians to help families. Patient support should include emotional support and clear communication of test results. Referral to a bereavement counselor, religious leader, peer support group, or mental health professional may be advisable for management of grief and depression. Feelings of guilt or anger in parents who have experienced a perinatal death are common and may be magnified when there is an abnormal child or a genetic defect. It is helpful to develop a standard protocol, particularly in units that rarely deliver fetal deaths (27). Patients should be offered the opportunity to hold their infants and to keep mementos such as pictures, foot and hand prints, and plaster casts. Some parents may welcome discussion and find relief in autopsy results. The results of the tests are important even when no specific diagnosis is identified. Patients should be allowed to make as many choices as possible regarding their experience. Prolonged hospitalizations are unnecessary and recovery on postpartum wards should be avoided.
Subsequent Pregnancy Management
The risk for virtually all adverse pregnancy outcomes are influenced by prior obstetric history and stillbirth is no exception. The recurrence risk for stillbirth is not well studied and reliable numbers for individual patients are often unavailable. A recent population-based study from Missouri noted stillbirth rate of 22.7 per 1,000 in women with prior stillbirth, representing an odd ratio of 4.7 compared with women without prior stillbirth (28). Increased recurrence risks were noted in African Americans (35.9/1,000) compared with whites (28). Counseling can be hampered by insufficient information regarding the etiology of the prior stillbirth. In many cases, the prior stillbirth may be unexplained despite a thorough evaluation. Following are the key components of management:
- Preconception or initial prenatal visit: detailed medical and obstetric history; evaluation and workup of previous stillbirth; determination of recurrence risk; smoking cessation; weight loss in obese women in preconception period; genetic counseling if family genetic condition exists; diabetes screen; thrombophilia workup – antiphospholipid antibodies only if specifically indicated; support and reassurance.
- First trimester: dating ultrasonography; first-trimester screen – pregnancy-associated plasma protein A, human chorionic gonadotropin, and nuchal translucency (29); support and reassurance.
- Second trimester: fetal ultrasonographic anatomic survey at 18-20 weeks of gestation; maternal serum screening (Quadruple) or single marker alpha fetoprotein if first trimester screening (29); support and reassurance.
- Third trimester: ultrasonographic screening for fetal growth restriction after 28 weeks of gestation; kick counts starting at 28 weeks of gestation. Multiple studies have demonstrated that women who report decreased fetal movement are at increased risk for adverse perinatal outcome (30). Although fetal kick counting is an inexpensive test of fetal well being, the effectiveness in preventing stillbirth is uncertain. Antepartum fetal surveillance starting at 32 weeks of gestation or 1-2 weeks earlier than prior stillbirth. It is noteworthy that in addition to recurrent pregnancy loss, prior stillbirth increases the risk for many obstetric complications, including intrauterine growth restriction, abruption, and preterm birth. The most commonly employed surveillance method is the non-stress test. Doppler velocimetry, amniotic fluid indexes, and serial ultrasonograms to assess placental function. Despite reassurances, the patient is likely to be anxious and to require ongoing support. Indeed, a large component of providing good care in subsequent pregnancies in women with prior stillbirth is to tend to patient’s emotional needs. Frequent visits, documentation of fetal heart tones and well being and lots of positive reinforcement are invaluable.
- Timing of delivery: the decision to proceed with early delivery to prevent stillbirth must incorporate an understanding of the increased risks of maternal and neonatal complications compared with potential benefits. Deliveries before 39 weeks of gestation are associated with increased risk of admission to neonatal special care units for respiratory complications and other neonatal morbidities. Most authors suggest elective induction at 39 weeks of gestation; if delivery before 39 weeks of gestation is decided the documented fetal lung maturity by amniocentesis is advised.
Summary
Despite improvements in antenatal and intrapartum care, stillbirth, defined as in utero fetal death at 20 weeks of gestation or greater, remains and important, largely unstudied, and poignant problem in obstetrics. More than 26,000 stillbirths were reported in the United States in 2004. Although several conditions have been linked to stillbirth, it is difficult to define the precise etiology in many cases. This review discusses known and suspected causes of stillbirth including genetic abnormalities, infection, fetal-maternal hemorrhage, and a variety of medical conditions in the mother. The proportion of stillbirths that have a diagnostic explanation is higher in centers that conduct a defined and systemic evaluation. In low-risk women with unexplained stillbirth the risk of recurrence stillbirth after 20 weeks of gestation is estimated at 7.8-10.5 per 1,000 births with most of this risk occurring before 37 weeks of gestation. The most prevalent risk factor associated with stillbirth is non-Hispanic black race, nulliparity, advanced maternal age, and obesity. The risk of subsequent stillbirth is twice as high for women with a prior live born, growth restricted infant delivered before 32 weeks of gestation than for women with a prior stillbirth. Amniocentesis for fetal karyotyping has the highest yield and is particularly valuable if delivery is not expected immediately.
In the second trimester, dilatation and evacuation can be offered. Labor induction also is appropriate at later gestational ages, if second trimester dilatation and evacuation is unavailable, or based on patient preference. Induction of labor with vaginal misoprostol is safe and effective in patients with a prior cesarean delivery with a low transverse uterine scar before 28 weeks of gestation. The optimal “workup” for stillbirth is uncertain. The most important test in the evaluation of a stillbirth is fetal autopsy; examination of the placenta, cord and membranes; and karyotype evaluation. Other tests to consider include Kliehauer-Betke, antibody screen, serology for syphilis, anticardiolipin antibodies, lupus anticoagulant screen, testing for heritable thrombophilias, urine toxicology screen and parvovirus serology. Subsequent pregnancies may be at increased risk for stillbirth and obstetric complications. Treatment of underlying medical care or obstetric conditions, antenatal surveillance, and induction of labor with fetal maturity may improve outcome. Patient support should include emotional support and clear communication of test results. Referral to a bereavement counselor, religious leader, peer support group, or mental health professional may be advisable for management of grief and depression.
Acknowledgment: Gratitude is expressed to Dr. Robert M. Silver, Professor of Obstetrics and Gynecology, Chief, Division of Maternal-Fetal Medicine, University of Utah Health Sciences Center, Salt Lake City, UT (USA) for contributions and helpful suggestions in preparing the manuscript. Special thanks to the Board of Directors for providing the funding for research and development.
Suggested Reading
- Stillbirth Collaborative Research Network (SCRN)
Research to determine the Extent and Causes of Stillbirth
https://scrn.rti.org/ - World Health Organization (WHO)
Stillbirths
http://www.who.int/reproductivehealth/topics/maternal_perinatal/stillbirth/en/
References
- Stanton C, Lawn JE, Rahman H, et al. Stillbirth rates: delivering estimates in 190 countries. Lancet 2006;367:1487-1494
- MacDorman MF, Hoyert DL, Martin JA, et al. Fetal and perinatal mortality, United States, 2003. Natl Vital Stat Rep 2007;55:1-17
- Silver RM. Fetal death. Obstet Gynecol 2007;109:153-167
- National Center for Health Statistics. State definitions and reporting requirements for live births, fetal deaths, and induced terminations of pregnancy. 1997 revision. Hyattsville (MD): NCHS; 1997. Available at: http://www.cdc.gov/nchs/data/misc/itop97.pdf Accessed 12 June 2012
- Silver RM, Varner MW, Reddy UM, et al. Work-up of stillbirth: a review of evidence. Am J Obstet Gynecol 2007:196(5):433-444
- Gardosi J, Kady SM, McGeown P, et al. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ 2005;331:1113-1117
- Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol 2005;193:1923-1925
- McClure EM, Nalubamba-Phiri M, Goldenberg RL. Stillbirth in developing countries. Int J Gynecol Obstet 2006;94(2):82-90
- Korteweg FJ, Bouman K, Erwich JJ, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol 2008;111:965-874. (Level III)
- Laury A, Sanchez-Lara PA, Peokowitz S, et al. A study of 534 fetal pathology cases from prenatal diagnosis referrals analyzed from 1989 through 2000. Am J Med Gent A 2007;143A:3107-3120. (Level III)
- American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin. Management of stillbirth. Number 102; March 2009. Obstet Gynecol 2009;113:748-761
- Reddy UM, Goldenberg R, Silver RM, et al. Stillbirth classification – developing an international consensus for research. Obstet Gynecol 2009;114:901-914
- Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol 2006;195:764-770. (Level II-3)
- Huang DY, Usher RH, Kramer MS, et al. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol 2000;95:215-221. (Level II-3)
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol 2003;189(3):861-873
- Hassan J, Connell J. Translational mini-review series on infectious disease: congenital cytomegalovirus infection: 50 years on. Clin Exp Immunol 2007;49(2):205-210
- Skjoldebrand-Sparee L, Tolfvenstam T, Papdogiannakis N, et al. Parvovirus B19 infection: association with third-trimester intrauterine fetal death. BJOG 2001;107(4):476-480
- Centers for Disease Control and Prevention (CDC). Congenital syphilis – United States, 2002. MMWR Morb Mortal Wkly Rep 2004;53(31):716-719
- Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008;47(4):554-566
- Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol 2005;193:1923-1935. (Level III)
- Wapner RJ, Lewis D. Genetics and metabolic causes of stillbirth. Semin Perinatol 2002;26:70-74
- Christiaens GC, Vissers J, Poddighe PJ, et al. Comparative genomic hybridization for cytogenetic evaluation of stillbirth. Obstet Gynecol 2000;96:281-286
- Korteweg FJ, Erwich HM, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol 2012;206:53.e1-12
- Tang OS, Lau WH, Chan CC, Ho PC. A prospective randomized comparison of sublingual and vaginal misoprostol in second trimester termination of pregnancy. BJOG 2004;111:1001-1005. (Level I)
- Dickinson JE. Misoprostol for second-trimester pregnancy termination in women with a prior cesarean delivery. Obstet Gynecol 2005;105:352-356. (Level II-2)
- Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical Foley catheter and the risk of uterine rupture. Obstet Gynecol 2004;103:18-23. (Level II-2)
- Leduc L, Farine D, Armson BA, et al. Stillbirth and bereavement: guidelines for stillbirth investigation. Maternal-Fetal Medicine Committee; Clinical Practice Obstetrics Committee. J Obstet Gynecol Can 2006;28:540-552. (Level III)
- Sharma PP, Salihu HM, Oyelesse Y, et al. Is race a determinant of stillbirth recurrence? Obstet Gynecol 2006;107:391-397
- Reddy UM. Prediction and prevention of recurrent stillbirth. Obstet Gynecol 2007;110:1151-1164
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med 2004;32:13-24
- Zhang X, Kraner MS. Variations in mortality and morbidity by gestational age among infants born at term. J Pediatr 2009;154:358-362, 362.e1
- Donovan EF, Besl J, Paulson J, et al. Ohio Perinatal Quality Collaborative. Infant death among Ohio resident infants born at 32 to 41 weeks of gestation, Am J Obstet Gynecol 2010;203:58.e1-5
- Halloran DR, Alexander GR. Preterm delivery and age of SIDS death. Ann Epidemiol 2006;16:600-606
- Rosenstein MG, Cheng YW, Snowden JM, et al. Risk of stillbirth and infant death stratified by gestational age. Obstet Gynecol 2012;120:76-82
Published: 23 November 2012
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


