Critical Care in Obstetrics: Disseminated Intravascular Coagulation Syndromes
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Disseminated intravascular coagulation (DIC) is a life-threatening situation that can arise from a variety of obstetrical and non-obstetrical causes. DIC is a syndrome that can be initiated by a myriad of medical, surgical, and obstetric disorders. Also known as consumptive coagulopathy, defibrination syndrome and generalized intravascular coagulation, it is not a disease per se, but rather a clinicopathologic syndrome that can be initiated by a myriad of underlying diseases, conditions, or disorders. Specific to the obstetric arena, some form of consumptive coagulopathy continues to be a concern after more than a century after DeLee's description of "temporary hemophilia" that developed in women with a placental abruption or a long-dead fetus in 1901. Ultimately, this clinicopathologic phenomenon culminates in a systemic intravascular activation of coagulation that completely disrupts natural hemostasis. In severe cases, this ineffective balance of natural anticoagulant mechanisms can result in widespread fibrin deposition leading to multi-organ failure. DIC is a common contributor to maternal morbidity and it is one of the leading causes for maternal mortality (1).
The purpose of this review is to discuss the pathophysiology of disseminated intravascular coagulation (DIC) syndromes, focusing on the triad represented by exaggerated activation of coagulation, consumption of coagulopathy, and impaired synthesis coagulation as well as anticoagulation proteins. The diagnosis of DIC with special attention to the available scores adding prognostic value to the laboratory parameters in patients with this dangerous condition or are at risk for its development are also reviewed. The principles of the treatment of DIC is discussed extensively from the literature. In recent years, novel diagnostic scores and treatment modalities along with bedside point-of-care tests were developed and may assist the clinician in the diagnosis and management of DIC. Team work and prompt treatment are essential for the successful management of patients with DIC.
Prevalence
The rate of DIC during pregnancy differ among cohorts and range from 0.03% to 0.35% (2). The reported incidence varies because of differing definitions and diagnostic criteria. To put this condition into the context of obstetric care in the United States, these authors reviewed delivery and postpartum hospitalization in 2-year increments over a decade from the Nationwide Inpatient Sample (3). From 1998 to 2009, they found that the prevalence of DIC had significantly increased from 9.2 to 12.5 per 10,000 delivery hospitalization - a 35% increase. Moreover, DIC complicating postpartum hospitalizations increased 83% over the same time period, 1.2 to 2.2 per 10,000 delivery hospitalizations. For the most recent 2-year period of 2010-2011, DIC was reported to be the second most common severe maternal morbidity indicator, 32 per 10,000 delivery hospitalizations. Even more importantly, DIC was associated with nearly one fourth of maternal deaths during this study period (3). Even so, DIC as the sole cause of maternal death is relatively uncommon and accounts for only 0.2% of pregnancy-related deaths in this country (4). In another way understanding these results, DIC is rarely the cause of death alone, but it is commonly associated with conditions leading to death. In this context, DIC is one of most important clinical conditions in terms of maternal morbidity and mortality in obstetrics.
Obstetric Causes of Disseminated Intravascular Coagulation (DIC)
DIC represents a life-threatening condition that is the endpoint of uncontrolled systemic activation of the hemostatic system, leading to a simultaneous wide-spread microvascular thrombosis that can compromise the blood supply to different organs and may lead to organ failure (5). This process is associated with increased degradation of coagulation factors as well as anticoagulation proteins and followed by their impaired synthesis, leading to uncontrolled bleeding. Acute, severe DIC is characterized by diffuse multiorgan bleeding, hemorrhagic necrosis, microthrombi in small blood vessels, and thrombi in medium and large blood vessels (6). This condition may occur in the setting of sepsis, major trauma, and obstetric calamities. The final scenario is represented by the exhaustion of coagulation / anticoagulation factors and platelets, leading to profuse uncontrollable bleeding and often death. In contrast to the acutely ill patient with complicated severe DIC, other patients may have mild or protracted clinical manifestations of consumption or even subclinical disease manifested by only laboratory abnormalities.
The clinical picture of subacute to chronic DIC is exemplified by the chronic hypercoagulability that may accompany malignancy, in particular with mucin-producing adenocarcinomas and acute promyelocytic leukemia. However, currently there are no reports in the literature regarding the occurrence of mild subacute DIC in pregnant women. The development of DIC as a result of predisposing conditions can be a life-threatening complication and is considered one of the leading causes for maternal morbidity and mortality worldwide. However, it is important to emphasize that DIC is not a disease by itself; it is always secondary to an underlying disorder that causes the uncontrolled activation of coagulation. The development of DIC during pregnancy can be either abrupt as in acute abruption or postpartum hemorrhage (PPH) or continuous as can be observed in a retained dead fetus. Of interest, obstetric complications such as placental abruption, amniotic fluid embolism, and acute fatty liver are associated with severe early-onset DIC that is accompanied by maternal coagulopathy. Obstetrical DIC has been associated with a series of pregnancy complications including the following (7):
- Acute peripartum hemorrhage (uterine atony, cervical and vaginal lacerations, and uterine rupture);
- Placental abruption;
- Preeclampsia / eclampsia / hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome;
- Retained stillbirth;
- Septic abortion and intrauterine infection;
- Amniotic fluid embolism; and
- Acute fatty liver of pregnancy.
Physiology and Pathogenesis
The DIC in obstetric hemorrhage activates coagulation and triggers fibrinolysis. Activation of fibrinolysis leads to the production of D-dimers and fibrin-degradation products. These will interfere with platelet function and can impair myometrial contractility (8). During pregnancy, a substantive increase in plasma volume is concomitantly augmented by production of most procoagulants (9). Importantly, fibrinogen (factor I) concentration increases approximately 50% above non-pregnant values, and during late pregnancy, it ranges from approximately 375 to 620 mg/dL. Thus, virtually all clotting factors and some of these are shown in the table below (10). At the same time, there is a reduction in levels of natural anticoagulants protein C and S and tissue factor pathway inhibitor-1 as well as an acquired resistance to protein C. In addition, profibrinolysin or plasminogen levels increase but there is also increased inhibition of fibrinolysis. As a result all of these alterations, the net result is that pregnancy is a procoagulants state.
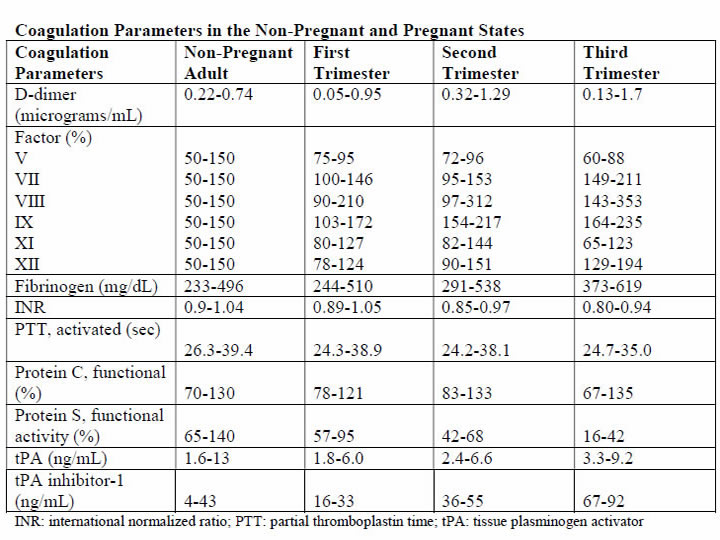
The current theory is that coagulation is primarily initiated by tissue factor, or thromboplastin, that forms complexes with factors VII and VIIa (11). Tissue factor is an integral membrane glycoprotein that is found in highly vascularized organ such as the brain, lungs and placenta, and it also can be expressed constitutively by certain cell types (12). In brief, the development of tissue factor FVIIa complexes ultimately generates activated factor X to initiate clotting, whereas the previously labeled "intrinsic" pathway is responsible for the amplification of this process. The end result of this coagulation process is fibrin formation, which is then counter-balanced by the fibrinolytic system, dedicated to the removal of excess fibrin. Augmented by thrombin to plasmin, it lyses fibrin and fibrinogen. The end result is production of fibrinogen-fibrin split products, which include D-dimers.
Endothelial dysfunction and platelet activation: Intact, dysfunctional or activated cells, as well as remnants of cell surfaces, inflammatory mediators, and coagulation proteins are all part of an interplay in which uncontrolled activation of coagulation cascade leads to DIC (13). Endothelial cells, platelets, but in some cases also leucocytes and cancer cells can participate in the genesis of the process leading to DIC by releasing proinflammatory cytokines, propagating the activation of coagulation on their surface or inducing tissue factor (TF) expression on their membrane. A systemic inflammatory response that is associated with markedly increased circulating proinflammatory cytokines such as tumor necrosis factor-α (alpha), interleukin-1 (IL-1), and interleukin-6 (IL-6) can lead to exaggerated expression of TF by leukocyte and endothelial cells. This will generate an uncontrolled coagulation response that will eventually deteriorate into DIC. Lastly, the initiation of coagulation leading to thrombin generation in DIC, is medicated by the TF/factor VIIa pathway, also known as the extrinsic coagulation pathway (6),(14). The most significant source of TF is not completely clear in all situations. TF may be expressed not only in mononuclear cells in response to proinflammatory cytokines (mainly IL-6) but also by vascular endothelial or cancer cells. Despite the potent initiation of coagulation by TF, the activation of coagulation cannot be propagated if physiological anticoagulant pathways function properly. However, in DIC all major natural anticoagulant pathways (i.e., antithrombin III, protein C system, and TF pathway inhibitor (TFPI) appear to be impaired (15). Plasma concentrations of antithrombin III, the most important inhibitor of thrombin, are markedly reduced during DIC because of a combination of consumption, degradation by elastase from activated neutrophils, and impaired synthesis.
A significant depression of the protein C system may further compromise an adequate regulation of activated coagulation (16). This impaired function of the protein C system is caused by a combination of impaired protein synthesis, cytokine-mediated down-regulation of endothelial thrombomodulin, and a fall in the concentration of the free fraction of protein S (the essential cofactor of protein C), resulting in reduced activation of protein C. Lastly, there seems to be misbalance of TFPI function in relation to the increased TF-dependent activation of coagulation. All these anticoagulant pathways are linked to the endothelium, and it is likely that endothelial cell activation and dysfunction are an important component of the imbalance between coagulation and anticoagulation systems. Of interest, experimental and clinical studies indicate that during DIC, the fibrinolytic system is largely suppressed at the time of maximal activation of coagulation (17).
During pregnancy maternal leukocytes are in a higher state of activation than in non-pregnant women and have characteristics akin to sepsis. However, they are well controlled during pregnancy, and it has been proposed that the trophoblast plays a role in the maintenance of the balanced systemic maternal inflammation during gestation. Nevertheless, in cases of sepsis caused by an infectious agent or septic abortion and at least in some of the cases of amniotic fluid embolism, this equilibrium is disturbed and the mother develops DIC (18).
Trophoblast properties and activation of the coagulation system: During normal gestation the trophoblast has two hemostatic functions - (a) to allow the laminar flow of maternal blood in the intervillous space and prevent it from clotting during the time; and (b) to prevent bleeding at the maternal fetal interface (5). The evidence supports the fact that any condition that disrupts the integrity of the trophoblast can lead to a release of large amount of potent TF that will activate the coagulation cascade and propagate an inflammatory response that can easily become systemic, leading to uncontrolled thrombin generation and the subsequent development of DIC (19). There are several conditions that are associated with DIC in which the current evidence suggests that the systemic maternal response is the result of endothelial activation. The classical one is abruption, especially that with concealed bleeding and fetal demise. These patients have a combination of consumption coagulopathy and discharge of thromboplastin (tissue factor) into the circulation (6).
Although the DIC developed in patients with placental abruption is regarded as a problem of consumption coagulopathy, it seems that there is more to it, meaning that often patients with a retroplacental clot have a much lower blood loss than those who developed PPH, yet the DIC in these patients is much more severe. A probable explanation is that this complication is associated with the release of procoagulating factors, such as thromboplastin, into the maternal circulation. In addition, local hypoxia and hypovolemia trigger endothelial response leading to increased expression of vascular endothelial growth factor, which causes an increased endothelial expression of TF (20). Of interest, if the abruption in concealed or it is severe enough to cause fetal demise, it is at much higher risk for the development of DIC because of a continuous release of TF in the maternal circulation. The probable mechanism leading to this observation is similar to that observed in amniotic fluid embolism with systemic release of TF that leads to systemic activation of coagulation and subsequent DIC.
Obstetrical Hemorrhage
Acute obstetrical hemorrhage is being considered by many as a leading cause for DIC. This form of consumption coagulopathy is classically related to PPH as a result of uterine atony, retained placenta, placenta accrete, or severe cervical or vaginal lacerations. In all of these cases, the mother is losing a large volume of blood and coagulation factors in a short time interval, and these patients are usually hemodynamically compromised. Currently, there is a debate whether this form of consumption coagulopathy is truly DIC or just a massive blood loss that depletes the patient's coagulation factors and can lead to death because of exsanguination. However, massive maternal bleeding may not be that straight-forward as a pure loss of coagulation factors. During the time of parturition and postpartum period, there is substantial activation of coagulation cascade and generation of thrombin as a result of release of TF to the maternal circulation following the separation of the membranes and the placenta (21). Thus, these women already have increased thrombin generation and indeed are regarded as high-risk patients for the development of deep vein thrombosis during the puerperium. The evidence brought herein, that parturients with PPH have a higher activation of coagulation cascade even above the physiological threshold, suggests that the clinicians who treat these patients must regard them as a high-risk group for DIC, even though the fundamental pathology is a rapid and massive loss of blood as well as coagulation factors. Therefore, patients with PPH need to be treated promptly, pharmacologically, and/or surgically and by blood products as well as volume expanders to sustain the maternal circulation and perhaps to prevent the subsequent development of DIC.
Amniotic Fluid Embolism
Amniotic fluid embolism remains one of the most devastating conditions in obstetric practice with an incidence of approximately 1 in 40,000 deliveries and a reported mortality rate ranging from 20% to 60% (22). The pathophysiology appears to involve an abnormal maternal response to fetal tissue exposure associated with breaches of the maternal-fetal physiologic barrier during parturition. This response and its subsequent injury appear to involve activation of proinflammatory mediators similar to that seen with the classic systemic inflammatory response syndrome. Data regarding the presence of risk factors for amniotic fluid embolism are inconsistent and contradictory; at present, no putative risk factor has been identified that would justify modification of standard obstetric practice to reduce the risk of this condition. Predisposing conditions are rapid labor; meconium-stained amniotic fluid; older maternal age; post-term pregnancy; labor induction or augmentation; eclampsia; cesarean, forceps or vacuum delivery; placental abruption or previa; and hydramnios. Associated uterine hypertonus appears to be an effect rather than a cause of amniotic fluid embolism.
The etiopathogenesis of amniotic fluid embolism is enigmatic. The prevailing theory is that TF from amniotic fluid and fetal squames in meconium initiate the profound systemic inflammatory response syndrome and DIC. Whatever the cause, the immediate response is pulmonary and systemic hypertension followed quickly by hypotension, hypoxia, and coagulopathy. Cardiac arrest typically follows and is a common cause of death. The reported frequency of fatal cases of symptomatic amniotic fluid embolism varies, but 60% or more is a reasonable estimate average (22). The syndrome of sudden peripartum hypoxia, cardiovascular collapse, and coagulopathy is real but has nothing to do directly with either amniotic fluid or embolization. The recovery of cellular debris of fetal origin from the maternal circulation may be a marker of this event but is neither sufficiently sensitive nor specific to be of diagnostic use because entry of amniocytes or other cells of fetal origin is commonly seen in normal pregnancy. This syndrome involves a series of recognizable clinical signs, symptoms and central hemodynamic changes similar to those seen in many cases of anaphylaxis or systemic inflammatory response syndrome (SIRS), and septic shock, suggesting the involvement of similar endogenous proinflammatory mediator and procoagulants activation or release in response to foreign antigenic substances.
The diagnosis of amniotic fluid embolism is a clinical one. Identification of elements of the classic triad of hypotension, hypoxia and coagulopathy and the careful exclusion of other conditions are essential for the diagnosis. The diagnosis is based on specific biochemical indices remains investigational. Several investigators have proposed the existence of more specific laboratory or autopsy findings to confirm the diagnosis of amniotic fluid embolism. These include various arachidonic acid metabolites, tryptase, urinary histamine, insulin-like growth factor binding protein-1, various markers of complement activation and postmortem immunohistologic staining for Sialyl Tn antigen, zinc coproporphyrin, or other evidence of pulmonary mast cell degranulation (24). Unfortunately, such studies are difficult to interpret; index cases are often chosen based on clinical findings, which were interpreted by the authors to be diagnostic of amniotic fluid embolism but which are generally reported in insufficient detail to allow the reviewer to be assured of the accuracy of the diagnosis. These problems are further compounded by the use of normal pregnant women as control participants rather than critically ill pregnant women in whom acute-phase inflammatory reactants would be expected to rise, regardless of diagnosis. Thus, although these descriptions of laboratory findings appear to be generally supportive of an inflammatory-based mechanism of disease in amniotic fluid embolism, to date none of these findings is of significant diagnostic or clinical use. Although the search for specific histologic or laboratory markers of amniotic fluid embolism continues, this condition remains primarily a clinical diagnosis of exclusion.
Management of amniotic fluid embolism includes immediate tracheal intubation with ventilator assistance, cardiopulmonary resuscitation, and other supportive measures. The latter includes improved oxygenation and support of the failing myocardium along with circulatory support. Because of bleeding from operative sites or lacerations and uterine atony, there is usually need for rapid blood and component replacement. The coagulopathy is especially problematic in women who have been or who are undergoing cesarean delivery. In undelivered women in whom cardiopulmonary resuscitation is necessary, consideration should be given for emergency cesarean delivery in an attempt to optimize these efforts. Perinatal outcomes are poor and inversely related to the maternal cardiac arrest-to-delivery interval (23). One of the most dramatic presentations of amniotic fluid embolism is the sudden development of cardiovascular collapse or cardiac arrest during pregnancy, most commonly during labor or immediately postpartum. Yet it is clear that not all cases of amniotic fluid embolism have cardiovascular collapse as their outstanding presenting symptoms. Amniotic fluid embolism can present as an isolated coagulopathy without cardiovascular collapse and insulin-like growth factor binding protein-1 can be used to confirm a diagnosis of amniotic fluid embolism (25).
HELLP Syndrome
It was once widely held that DIC was a fundamental feature of the preeclampsia syndrome. This belief was stimulated by findings of thrombocytopenia and microangiopathic hemolysis as well as massive fibrin deposition found at autopsy in women with severe preeclampsia and eclampsia. It was also known at that time that some degree of hepatocellular necrosis was commonly found in women dying of eclampsia. Soon thereafter is was discovered that the serum analytes for hepatic transaminases-aspartate and alanine transferase, were directly related to the presence of hepatocellular necrosis. This led Weinstein in 1982 to coin the term HELLP syndrome, H=hemolysis, EL=elevated liver enzymes, and LP=low platelet count (26). The mild-to-moderately increased levels of fibrin split products in some women, along with slightly elevated levels of thrombin-antithrombin complexes are indicative of some increased intravascular coagulation (26). It follows that treatment is not necessary for the mild DIC associated preeclampsia, eclampsia, or HELLP syndrome. One exception is the occasional woman who will need platelet transfusion for troublesome bleeding resulting from thrombocytopenia and platelet dysfunction at the time of cesarean delivery. Other exceptions are women who have a concomitant placental abruption, acute fatty liver disease, or dilutional coagulopathy from major hemorrhage.
One of the major differences between acute fatty liver of pregnancy and HELLP syndrome is the prevalence of DIC. In this study (27), DIC was present in more than 70% of patients with acute fatty liver of pregnancy and less than 15% of those with HELLP syndrome. Thus, although women with HELLP syndrome have a reduced production of fibrinogen and other coagulating as well as anticoagulation factors that can lead to the development of DIC, this is not the central feature of this disease. From the evidence brought herein, DIC is a central feature of acute fatty liver and in a way reflects the severity of the hepatic injury, whereas in HELLP syndrome, it is present in only a fraction of the patients, probably those with more severe form of microangiopathic hemolytic anemia associated with this syndrome.
Acute Fatty Liver of Pregnancy
Acute fatty liver of pregnancy is a rare, an estimated incidence between 6 and 14 per 100,000 pregnancies, a potentially fatal complication of pregnancy (28). It is characterized by fatty microvascular infiltration of hepatocytes with progressive loss of liver function, without alteration of the overall structure of liver. Women who develop this complication have abnormal renal function and DIC. The mechanisms by which DIC develops is this complication is a combination of reduced liver production of fibrinogen as well as coagulation proteins and hemorrhage. Evidence in support of this view for their hemostatic condition was classified, in this study (29), according to the International Society of Thrombosis and Haemostasis DIC score, and 80% of these women had unequivocal DIC defined as composite score of 5 or greater. Women who developed DIC had abnormally low plasma fibrinogen concentrations and persisted for the first several days after delivery along with only mild to moderately elevated fibrin degradation products. At the same time, there was also evidence for continuing increased procoagulants consumption caused by ongoing DIC provided by the modestly levels of fibrin degradation products in the face of depressed plasma fibrinogen concentrations. This observation was in contrast to that of patients with abruption in whom the fibrinogen concentration recovered into normal range several hours after the acute event. Collectively the continuous low fibrinogen concentration and abnormal function concentration and abnormal function of the coagulation cascade is the result of liver dysfunction associated with acute fatty liver of pregnancy, leading to a lower production of coagulation factors, anticoagulation proteins, and fibrinogen by the liver.
Sepsis Syndrome
The sepsis syndrome is induced by a systemic inflammatory response to bacteria or viruses or their by-products such as endotoxins and exotoxins. CD4 T cells and leukocytes are stimulated to produce proinflammatory compounds that include tumor necrosis factor-α, several interleukins, other cytokines, proteases, oxidants, and bradykinin that result in a "cytokine storm" (30). Many other cellular reactions then follow that include stimulation of proinflammatory and anti-inflammatory compounds, procoagulants activity, gene activation, receptor regulation, and immune suppression. At the same time, endotoxin stimulates endothelial cells to upregulate TF and thus procoagulants production while it decreases the anticoagulant action of activated protein C. The severity of the sepsis syndrome is a spectrum, and the mortality rate in non-pregnant patient is 20% to 35% with severe sepsis and 40% to 60% with septic shock. According to the Centers for Disease Control and Prevention, sepsis caused 4.2% of pregnancy-related deaths in the United States from 2006 to 2010. The most common cause in pregnancy is uro-sepsis from pyelonephritis caused by Escherichia Coli and Klebsiella species. There are also potent bacterial exotoxins that can cause severe sepsis syndrome - Clostridium perfringens toxic shock syndrome, toxin-1 producing Staphylococcus aureus, and toxic shock-like exotoxin from group A β-hemolytic streptococci. These exotoxins cause rapid and extensive tissue necrosis and gangrene, especially of the postpartum or post-abortion uterus, and they may cause profound cardiovascular collapse. Exotoxins are also potent inducers of DIC, and some of the effects of clostridial sepsis on platelets, fibrinogen, and fibrin split products.
Because of the high associated mortality rate, in 2004, a consensus effort was launched as the Surviving Sepsis Campaign (31). The cornerstone of management is early goal-directed management and prompt recognition of serious bacterial infection and close monitoring of vital signs and urine flow. The campaign calls for three basic steps to be performed as simultaneously as possible: (a) evaluation of the sepsis source and its sequelae; (b) assessment of cardiopulmonary function; and (c) immediate management. The most important step in sepsis management is rapid infusion of 2L and sometimes as many as 4-6L of crystalloid fluids to restore renal perfusion in severely affected women. Simultaneously, appropriately chosen broad-spectrum antimicrobials are begun. In anemia coexists with severe sepsis, blood is given along with crystalloid to maintain the hematocrit at approximately 30%. In general, with sepsis, correction of all facets of DIC is not necessary as long as the patient is not bleeding. Because continuing sepsis may prove fatal, debridement of necrotic tissue or drainage of purulent material is crucial. Some examples are uterine curettage for septic abortion, hysterectomy for a necrotic uterus, ureteral catheterization for obstructive pyelonephritis, and debridement for necrotizing fasciitis.
Fetal Death and Delayed Delivery
DIC with prolonged retention of a dead fetus is unusual today because fetal demise is easily confirmed by ultrasonography, and there are highly effective methods for pregnancy termination on its discovery. The pathogenesis of this coagulopathy is thought to be mediated by the slow release of TF or thromboplastin from the dead fetus and the placenta (32). Currently, the syndrome is only occasionally encountered in twin or triplet pregnancy in which there is one dead co-fetus with one or two surviving fetuses in an intact and ongoing pregnancy. It is also encountered in women with missed abortion of several weeks' duration. If the surviving fetus is delivered during this time when there is a clinical coagulopathy, treatment of the coagulopathy may be necessary if cesarean delivery is undertaken or if severe lacerations are incurred.
Diagnosis of DIC
In the order of relative importance in patients with DIC, the tests are platelet count (decreasing), prothrombin time (PT) prolongation, fibrin-related marker (increasing), and fibrinogen (decreasing). One key message is that the tests should be repeated to reflect the dynamic changes on the basis of laboratory results and clinical importance. Thrombocytopenia is the most common laboratory diagnostic feature for DIC (33). However, the platelet count may not always be very low and may even be in normal range in patients who have recently started developing DIC. On the other hand, the gestational thrombocytopenia of the third trimester may confuse the thrombocytopenia of DIC. To determine whether the thrombocytopenia is due to DIC, a downward trend in the platelet count is most important, even if the count remains in the normal range. A diagnosis of DIC is often considered when the PT and activated partial thromboplastin (APTT) are considerably prolonged. However, in pregnancy women, normal ranges for these tests are considerably shorter than that for the general population. Prolongation of PT and APTT may not occur until underlying condition has progressed considerably. This is especially true in the case of APTT, which can be shortened because of increased concentrations of factor VIII seen in pregnancy. It is important that in the correct clinical context, attention is paid to prolongation of the PT and APTT, even in the normal range, because suggestive of thrombin generation and clinical worsening and treatment commenced when they are even mildly prolonged.
The acutely bleeding patient does not need any score evaluation but prompt infusion of blood products according to preexisting protocols. However, in many cases DIC develops gradually and through different underlying mechanisms. In the latter group, using such a scoring system can alert the clinician that the patient is deteriorating and needs further attention and treatment. In addition, the introduction of such a scoring system into clinical work will help to validate the diagnosis and create a common language between clinician and researchers that will assist in the promotion of the understanding and management of DIC during pregnancy. The first DIC score has been recommended by the International Society for Thrombosis and Haemostasis (ISTH) in 2001, showing a correlation between key clinical observations and outcomes (34). Using the same parameters, the Japanese Association for Acute Medicine (JAAM) published an additional score in 2005, offering a good predictive value for the diagnosis of DIC and the identification of critically ill non-pregnant patients (35). These scores can be used not only as a diagnostic but also as a prognostic tool. Thus, in the non-pregnant state, a DIC is important in the diagnosis of patients with DIC and carries a diagnostic and prognostic value. Because the physiological hemostatic changes occurring in pregnancy affect the application of these scores to gestation, an adjusted score for the pregnant state was needed. Based on this consideration, a pregnancy modified DIC score by using only 3 components of the ISTH DIC score (platelet count, fibrinogen concentrations, and the PT difference) with an area under the curve of 0.975 (P<.001) and at a cutoff of 26 or more points has a sensitivity of 88% and a specificity of 96% for the diagnosis of DIC. At this cutoff the pregnancy-modified DIC score showed a positive likelihood ratio of 22 and a negative likelihood ratio of 22 and a negative likelihood ratio of 0.125 (2),(6).
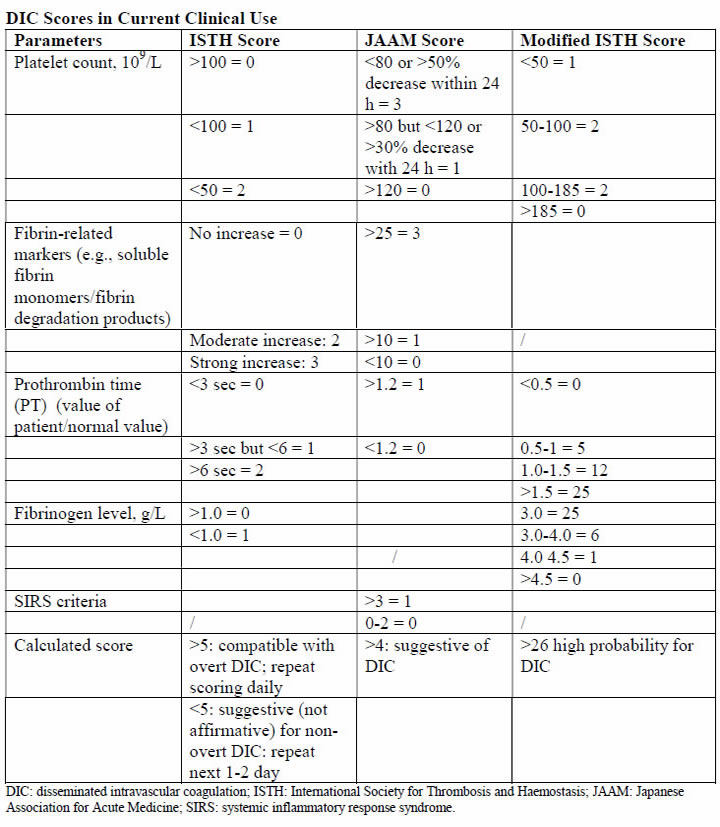
Some studies reported the utility fibrinogen/C-reactive protein ratio as a diagnostic and prognostic tool for the development of overt DIC in patients with underlying HELLP syndrome (36). Their population was divided in two groups because the diagnosis was based on the ISTH criteria. They also used D-dimer concentration. This study showed that the fibrinogen/C-reactive protein could differ significantly between patients who develop DIC and those who do not, showing to be a better indicator for predicting a DIC than is the fibrinogen level. The applicability of this parameter into clinical practice is limited by the very small number of patients included who developed the studied condition. It is not advisable to use coagulation parameters of non-pregnant patients in comparison with pregnant patients, because curves are different and point-of-care tests perform differently during pregnancy, parturition, and puerperium.
Management of DIC
Managing the underlying condition that predisposes to DIC: it is an intermediary mechanism of disease and is always secondary to an underlying process. This hypothesis is supported by fact that the mortality of DIC associated with placental abruption is less than 1%, whereas that associated with sepsis related to obstetrical cases is 50-80% (37). For this reason, appropriate management of the primary condition is paramount in limiting the excess thrombin generation.
Supportive care with blood products and related measures: the term, consumption coagulopathy, often classically used for DIC, suggests the need for replacement of blood components in the management of DIC. The following principles are considered in this context: in relation to the coagulation factors, their reduction has to be considered in relation to their hemostatic levels, especially if the patient is bleeding in the case of platelets, because an element of platelet dysfunction exists, this may contribute to the bleeding; fibrinogen as an acute-phase reactant has recently been shown to play in important role in hemostasis in obstetrics; an adequate hemoglobin concentration needs to be maintained because severe anemia can worsen the rheology by being unable to push platelets toward the endothelium and also reducing oxygen delivery to already compromised tissues.
The thresholds for transfusing blood components are those recommended by the harmonized guidance from the ISTH, except in case of fibrinogen. In bleeding patients or those who need interventions, this should be started in the following conditions; fibrinogen less than 1.5 g/L (2 pools of cryoprecipitate should raise the fibrinogen level by 1.0 g/L in the absence of continued bleeding; if available, fibrinogen concentrate may be used); platelets less than 50 X 109/L (1-2 adult doses of platelets), but if ongoing hemorrhage is occurring, a higher threshold of 75 X 109/L may be a starting point; PT and APTT prolonged outside normal ranges (15-30 mL/kg of fresh frozen plasma, higher volumes likely to replace more coagulation factors and is preferred if volume overload is not a concern). The evidence on different recommendation transfusion protocols for blood products is available in the literature, below is massive transfusion protocol used at Parkland Health and Hospital System (38):
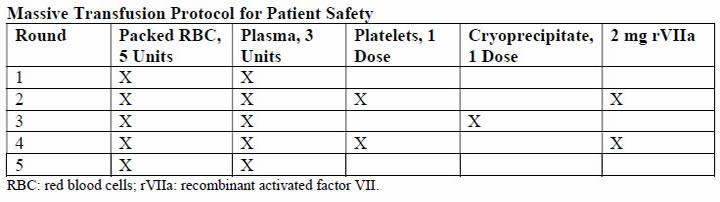
A principal concern raised with the use of rVIIa is relatively high frequency of thromboses - both arterial and to lesser degree, venous thrombosis. There is also concern regarding its effectiveness (39). At this time, although still used for massive hemorrhage, there is insufficient clinical evidence to make firm recommendations on the obstetric use of rVIIa for the treatment of DIC. Some guidelines suggest the use of rVIIa use before proceeding to hysterectomy for massive PPH. Tranexamic acid has had resurgence in recent years for the management of massive hemorrhage. In the obstetrical setting, the multinational World Maternal Antifibrinolytic Trial (WOMAN) trial is likely to improve the evidence regarding this issue (40). The updated World Health Organization guidelines for PPH treatment state a possible use of tranexamic acid in case other measure (uterotonics, uterine message, fluid resuscitation) fail, and this is suggested in the United Kingdom as "recommended for consideration" in cases of intractable PPH (41).
Regular clinical and laboratory surveillance: because DIC is a dynamic process, close monitoring of the patient is crucial to evaluate clinical improvement or worsening, to assess whether the process of DIC is abated, and notice the development of complications including organ failure, allowing prompt intervention. Repeated monitoring of the laboratory values including full blood count for hemoglobin and platelet count and clotting screen including fibrinogen is needed.
Seeking assistance from the relevant specialists at the earliest: DIC is a complex condition in which dysfunction in multiple organ systems contributes to the pathophysiology, and as such, the obstetrician should not be left alone in managing the patients but he or she should be involved as the part of a group of physicians from different specialties. As a consequence, attending surgeons, intensive care specialists, and hematologists, but also all the people in patient care, including nurses and support staff, must take part in the case management.
Algorithm summarizing diagnosis and treatment of DIC
- Acute peripartum hemorrhage;
- Placental abruption;
- Preeclampsia/eclampsia/HELLP syndrome;
- Retained stillbirth;
- Septic abortion and intrauterine infection;
- Amniotic fluid embolism;
- Acute fatty liver of pregnancy.
If clinical signs of DIC are present:
- Perform blood sample for laboratory testing;
- Start supportive care with blood products and related measures without waiting for laboratory results;
- Generate the multidisciplinary approach;
- Keep clinical and laboratory surveillance.
If clinical signs of DIC are not present:
- Perform classical laboratory evaluation;
- Point of care testing;
- Scoring evaluation;
For high-risk patients for DIC: consider early supportive measures; involve the multidisciplinary team; and keep clinical and laboratory surveillance.
For low-risk patients for DIC: keep clinical and laboratory surveillance.
Summary
DIC is a life-threatening situation that can arise from a variety of obstetrical and non-obstetrical causes. Prompt diagnosis and understanding of the underlying mechanisms of disease leading to this complication is essential for favorable outcome. In recent years novel diagnostic scores and treatment modalities along with bedside point-of-care tests were developed and may assist the clinician in the diagnosis and management of DIC. Team work and prompt treatment are essential for the successful management of patients with DIC. Early and accurate recognition of DIC is the hallmark of success in the treatment of this dire complication. There is no single laboratory or clinical test that is sensitive and specific enough to diagnose DIC. Also, the effect of the pathologies on the coagulation profile of the patients cited previously and the risk to develop DIC is not evident in all cases. For these reasons, and the need to provide the clinician a tool for early identification of DIC, efforts have been made to create scoring systems to identify patients high risk for this dangerous complication. All those scores use simple and readily available coagulation tests including platelet count, prothrombin time prolongation, fibrinogen, and fibrin split products/D-dimer concentrations. The management of DIC in obstetrics remains a major clinical challenge. The inciting disease-specific syndrome may be complex and require directed management strategies for correction of the underlying disorders. Equally important is treatment of frequently concomitant massive blood loss that worsens the coagulopathy. With limited clinically proven management strategies available, the need for future studies is obvious. We look forward to these studies designated to address our numerous evidence-based deficits, especially regarding management of obstetric DIC syndromes.
References
- Walfish M, Neuman A, Wlody D. Maternal haemorrhage. Br. J Anaesth 2009;103(Suppl. 1):147-156
- Erez O, Novack L, Beer-Weisei R, et al. DIC score in pregnant women - a population based modification of the International Society on Thrombosis and Hemostasis score. PLoS One 2014;9:e93240
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery, postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029-1036
- Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006 - 2010. Obstet Gynecol 2015;125:5-12
- Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev 2009;23:167-176
- Levi M, van der Poll T. Disseminated intravascular coagulation: a review for the internist. Intern Emerg Med 2013;8:23-32
- Erez O, Mastrolia SA, Thachil J. Disseminated intravascular coagulation in pregnancy: insights in pathophysiology, diagnosis and management. AJOG 2015;213:452-463
- Dalainas I. Pathogenesis, diagnosis, and management of disseminated intravascular coagulation: a literature review. Eur Rev Med Pharmacol Sci 2008;12:19-31
- Kenny L, McCrae K, Cunningham FG. Platelets, coagulation, and the liver. In: Taylor R, Roberts JM, Cunningham FG, editors. Chesley's hypertension in pregnancy. 4th ed. Amsterdam (the Netherlands): Academic Press; 2014
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians [published erratum appears in Obstet Gynecol 2010;115:387]. Obstet Gynecol 2009;114:1326-1331
- Levi M, Seligsohn U. Disseminated intravascular coagulation. In: Kaushansky K, Lictman M, Beutler, et al, editors. Williams hematology. 8th ed, New York (NY): McGraw-Hill; 2010. P.2101
- Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost 2006;32:11-23
- Anas AA, Wiersinga WJ, de Vos AF, et al. Recent insights into the pathogenesis of bacterial sepsis. Neth J Med 2010;68:147-152
- Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010;38:S26-34
- Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med 2001;29:S90-94
- Choi Q, Hong KH, Kim JE, et al. Changes in plasma levels of natural anticoagulants in disseminated intravascular coagulation: high prognostic value of antithrombin and protein C in patients with underlying sepsis or severe infection. Ann Lab Med 2014;34:85-91
- Levi M. Disseminated intravascular coagulation. Crit Care Med 2007;35:2191-2195
- Romero R, Kadar N, Vaisbuch E, et al. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol 2010;64:113-125
- Erez O, Gotsch F, Mazaki-Tovi S, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med 2009;22:672-687
- Krikun G, Huang ST, Schatz F, et al. Thrombin activation of endometrial endothelial cells: a possible role in intrauterine growth restriction. Thromb Haemost 2007;97:245-253
- Rath WH. Postpartum hemorrhage: update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand 2011;90:421-428
- Clark S. Amniotic fluid embolism. Obstet Gynecol 2014;123:337-348
- Kramer MS, Rouleau J, Liu S, et al. Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic fluid embolism: incidence, risk factors and impact on perinatal outcomes. BJOG 2012;119:874-879
- Hasegawa a, Murakoshi T, Otsuki Y, et al. Clinical course of disseminated intravascular coagulopathy-type amniotic fluid embolism: a report of three cases. J Obstet Gynecol Res 2016;42:1881-1885
- Benson MD. What is new in amniotic fluid embolism? Obstet Gynecol 2017;129:941-942
- Cunningham FG, Nelson DB. Disseminated intravascular coagulation syndromes in obstetrics. Obstet Gynecol 2015;126:999-1011
- Vigil-De Garcia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet 2001;73:215-220
- Nelson DB, Yost NP, Cunningham FG. Hemostatic dysfunction with acute fatty liver of pregnancy. Obstet Gynecol 2014;124:40-46
- Bkhtian K, Meijeris JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med 2004;32:2416-2421
- Russell JA. Management of sepsis. N Engl J Med 2006;355:1699-1713
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327
- Eddib A, Rodgers B, Lawler J, et al. Monochorionc pseudo-monoamniotic twin pregnancy with fetal demise of one twin and development of maternal consumptive coagulopathy. Ultrasound Obstet Gynecol 2006;28:736-437
- Levi M, Meijers JC. DIC: which laboratory tests are most useful? Blood Rev 2011;25:33-37
- Taylor FB, Toh CH, Hoots WK, et al. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86:1327-1330
- Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 2006;34:625-631
- Windsperger K, Lehner R. The fibrinogen/CPR ratio as a new parameter for the diagnosis of disseminated intravascular coagulation in patients with HELLP syndrome and as a predictive factor for neonatal outcome. Am J Obstet Gynecol 2013;208:118.e1-7
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haemotology. Br. J Haematol 2009;145:24-33
- Shields LE, Wiesner S, Fulton J, et al. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol 2015;212:272-280
- Pacheco LD, Saade GR, Gei AF, et al. Cutting-edge advances in the medical management of obstetrical hemorrhage. Am J Obstet Gynecol 2011;205:526-532
- Sentilhes L, Lasocki S, Ducloy-Bouthors AS, et al. Tranexamic acid for the prevention and treatment of postpartum haemorrhage. Br J Anaesth 2015;114:576-587; available at http://www.thewomantrial.lshtm.ac.uk Accessed on 2 July 2017
- Royal College of Obstetricians and Gynecologists. Prevention and management of postpartum haemorrhage. Guideline no. 52. Revised April 2011. London: Royal College of Obstetricians and Gynaecologists; 2009
Published: 13 July 2017
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


