Вирусная инфекция Зика при беременности
Бюллетень WHEC Практика и клинической управления для медицинских работников. Образовательный грант предоставляемых Здоровье женщин и центр образования (WHEC).
Zika virus was first identified from the Rhesus monkey in 1947 in Zika Forest, Uganda. The virus was subsequently isolated from humans 5 years later (1). Last February, an epidemic of a rash-associated mild viral illness rapidly spread through a number of northeastern Brazilian states. By May, the illness was confirmed to be due to the mosquito-borne Zika virus. On 11 November 2015, the Brazilian Ministry of Health declared a public emergency precipitated by reports of a 10-fold higher rate of fetal microcephaly occurring in these same Zika-affected states. On 17 November 2015, the Brazilian Ministry of Health reported that real-time reverse transcription polymerase chain reaction (rRT-PCR) analysis of amniotic fluid obtained from two women with microcephalic fetuses demonstrated the presence of Zika virus ribonucleic acid (RNA). The same day the World Health Organization (WHO) issued an epidemiological alert regarding the increased occurrence of microcephaly in northeast Brazil. A few days later health authorities in French Polynesia reported at least 17 cases of fetal or infant central nervous malformations associated with an earlier outbreak of Zika virus. In December 2015, the Centers for Disease Control and Prevention (CDC) in the United States, reported a single case of Zika virus infection in Puerto Rico and on 15 January 2016, the CDC issued a travel advisory to pregnant women recommending they postpone travel to Mexico, Puerto Rico, and parts of Central America and South America due to the presence of the Zika virus. On 22 January 2016, the advisory was expanded to include parts of the Caribbean and Polynesia. In El Salvador, health officials went so far as to recommend that women postpone becoming pregnant until 2018.
The purpose of this document is to discuss effects of Zika virus infection in pregnancy, review the evidence for causality of fetal anomalies and prevention. Although Zika was documented more than 60 years ago, because adverse pregnancy or birth outcomes have not been previously reported, research into perinatal infection and transmission is limited. The risk of microcephaly among infants of mothers infected by Zika is estimated to be between 0.9% and 13%. Breastfeeding has not been identified as a mode of transmission. This review presents new information on the broadened spectrum of anomalies now known to be causally related to congenital Zika virus infection and on the increasing number of serious neurologic complications directly related to Zika virus infection in adults. We have also updated the recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of Zika virus infection.
Zika Virus
Key Facts:
- Zika virus disease is caused by a virus transmitted primarily by Aedes mosquitoes;
- People with Zika virus disease can have symptoms including mild fever, skin rash, conjunctivitis, muscle and joint pain, malaise or headache. These symptoms normally last for 2-7 days;
- There is scientific consensus that Zika virus is a cause of microcephaly and Guillain-Barre syndrome (GBS). Links to other neurological complications are also being investigated. Figure
Figure 1b. Transmission is caused via mosquitoes.
The increase in travel has also permitted rapid spread of infected mosquitoes across continents. In the past, the slave trade which shipped people from Africa to South America brought yellow fever to South America. The International Health Regulations have always recommended special precautions such as disinfection in planes to reduce transportation of mosquitoes between countries. Despite these precautionary measures, Aedes species have established themselves in environments and regions well beyond their original tropical forest ecological niche. Aedes aegypti could be called the 'dogs' of the mosquito world because they have become so 'domesticated' that almost every aspect of their biology is dependent on humans. They prefer to live in and around human homes. Many are born, breed and die in human homes, and biting aggressively, during daylight hours. A female will bite several people before she considers her blood meal adequate for egg-laying. Another Aedes species able to transmit flavirviruses, Aedes albopictus, has the advantage of producing eggs able to survive in cooler temperatures so they can live in more temperate regions. As a result Aedes are competent urban vectors for not only Zika virus but other flaviviruses such as yellow fever. With ever greater urbanization and climatic phenomena (the El Nino effect and global warming) producing warmer and wetter environments, these mosquito species are thriving. As their numbers rise, so too does the probability that more humans will be bitten by those carrying flaviviruses. The current Zika virus and yellow fever epidemics are a symptom of the increased risk of vector-borne epidemics due to much greater vector density, especially in urban settings (2).
Brazil has seen a concomitant increase in the frequency of neonatal microcephaly (3). These parallel findings suggested a link between Zika and birth defects – a relationship not previously identified. With the outbreak of Zika infection and its probable link to teratogenicity has come vast media attention. Zika was declared a Public Health Emergency of International Concern by the World Health Organization (WHO) in February 2016 and it is predicted that transmission to new countries will continue during the upcoming months to years (4). In June 2016, the WHO took the momentous step of recommending that women living in Zika-endemic areas delay child-bearing.
List of Countries and territories reporting mosquito-borne Zika Virus Transmission
70 Countries and territories have reported evidence of mosquito-borne Zika virus transmission since 2007 (67 since 2015): 53 with a first reported outbreak from 2015 onward; 4 with having possible endemic transmission or evidence of local mosquito-borne Zika infections in 2016; and 13 with evidence of local mosquito-borne Zika infections in or before 2015, but without documentation of cases in 2016, or with the outbreak terminated (5). See map below
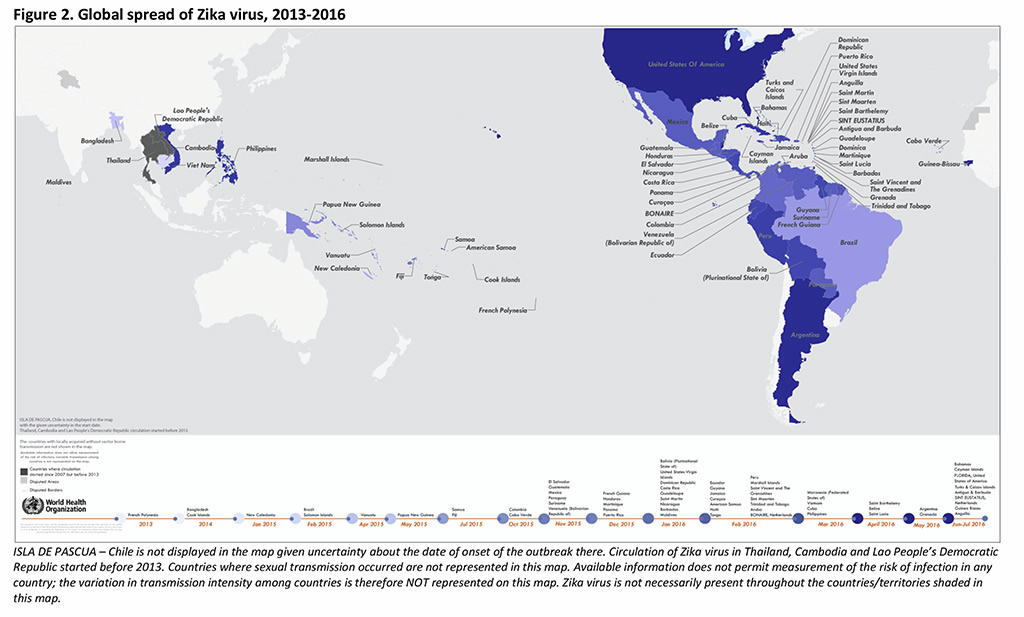
Key Updates:
- Countries and territories reporting mosquito-borne Zika virus infections for the first time: Bahamas;
- Countries and territories reporting microcephaly and other central nervous system (CNS) malformations potentially associated with Zika virus infection for the first time: Honduras and Suriname;
- Countries and territories reporting Guillain-Barré syndrome (GBS) cases associated with Zika virus infection for the first time: Costa Rica and Guatemala;
- Operational measures from the WHO Eastern Mediterranean Region: WHO will conduct Zika risk assessment missions including to Somalia, WHO is planning a training workshop on Incident Command System with partners in addition to a workshop to develop surveillance and guidance for detection of Zika and other arboviral diseases, both for November 2016; and WHO is rolling out three training workshops on prevention and control of Aedes mosquitoes for national entomologists from August to October.
Zika Virus Infection in United States
The Florida Department of Health has identified two areas of Miami-Dade County where Zika is being spread by mosquitoes. In addition to the previously identified area in the Wynwood neighborhood, there is now mosquito-borne spread of Zika virus in a section of Miami Beach. The CDC guidance is for people who live in or traveled to the identified area of Miami Beach any time after 14 July 2016. This guidance also still applies for those who live in or traveled to the previously identified Wynwood area any time after 15 June 2016. These time-frames are based on the earliest time symptoms can start and the maximum 2-week incubation period for Zika virus. Please review map available: https://www.cdc.gov/zika/intheus/maps-zika-us.html
Vector and Disease Features
Zika is transmitted to humans via the Aedes species of mosquitoes. These mosquitoes are also responsible for the transmission of dengue fever and chikungunya viruses. The Aedes mosquitoes are unique; unlike other species of mosquitoes, they are aggressive daytime biters. The primary species transmitting Zika to humans is the Aedes aegypti and Aedes albopictus have been found in the United States, primarily in the southeastern region. Aedes albopictus is the vastly more prevalent species. The most common symptoms of Zika virus infection are acute onset of fever, maculopapular rash, joint pain, and conjunctivitis. Other symptoms include muscle pain and headache.
Zika is a flavivirus, a single-stranded RNA virus (6). Zika virus is challenging to identify and therefore challenging to study for several reasons. First, only 1 in 5 people infected with the virus will become ill (3). Not only are disease symptoms usually mild, they can be identical to many other common mosquito-transmitted viruses. Because mild viral illnesses are ubiquitous in many of the regions affected with Zika, people may never realize they have been infected. In patients who do experience symptoms will occur between 3 and 12 days after being bitten by an infected mosquito and will last between 2 and 7 days (3,6). Symptoms include mild fever, maculopapular rash, headache, arthralgia, and myalgias. One distinctive feature of Zika in non-purulent conjunctivitis; while this complaint is also found among patients with dengue fever and chikungunya, its presentation seems to be much more common and severe in Zika cases (7). Zika has not been shown to cause hemorrhagic fever, and disease requiring hospitalization and/or resulting in death is rare. As in the case of other viral illnesses, once infected, individuals are protected from future infection (1).
Modes of Transmission
Through Mosquito Bites
- Zika virus disease is caused by a virus transmitted by Aedes mosquitoes;
- The virus is known to circulate in Africa, the Americas, Asia and the Pacific.
Mother-to-Child Transmission
Although Zika was discovered more than 60 years ago, because adverse pregnancy or birth outcomes have never been previously reported, research into perinatal infection and perinatal transmission is limited and ongoing. Two theories of perinatal transmission exist. The first is transplacental transmission. This theory proposes that virus is transferred directly from the mother to the fetus via the placenta. Once infected, the fetus suffers neural damage as a direct result of the virus. The other theory is one of placental inflammation, which proposes that maternal viral infection creates a placental inflammatory response. This response in turn results in fetal neural damage. The former theory is similar to other documented models of viral transmission and poor outcomes and is the more widely accepted view (8).
Sexual Transmission
A person with Zika can transmit the virus to his or her partner(s) through vaginal, anal, and oral sex. The sharing of sex toys may also put someone at risk. Zika has been detected in semen, vaginal fluids, saliva, urine, and breast milk. There is no evidence at present that Zika can be transmitted through saliva during deep kissing. There is documented evidence of sexual transmission of Zika from male-to-female, male-to-male and female-to-male sex partners. Female-to-female sexual transmission has not yet been reported but it is biologically plausible.
WHEC recommends:
- All pregnant women with sex partners who live in or traveled to an area with Zika use barrier methods during sex or abstain from sex for the remainder of their pregnancy;
- All other couples in which a partner has been in an area with Zika can also reduce the risk of sexual transmission by using barrier methods or abstaining from sex: Barriers include male and female condoms and dental dams and to be effective, barrier methods must be used from start to finish, every time during vaginal, anal or oral sex.
- Healthcare providers should: Test all pregnant women who may have been exposed to Zika sexually (i.e., had sexual contact without a barrier method with a person who lives in or has traveled to an area with Zika) and test any patients for Zika if they develop symptoms of Zika and report potential sexual exposure to a partner who lives in or traveled to an area with Zika.
- Independently of considerations regarding Zika virus, WHEC always recommends the use of safer sexual practices including correct and consistent use of condoms to prevent HIV, other sexually transmitted infections and unwanted pregnancies.
Presence of Zika virus in other body fluids: Publications on the presence of Zika virus in other body fluids that may be involved in sexual transmission have also been considered. Studies have reported the presence of Zika virus by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) in saliva and urine (9). The persistent shedding of Zika virus ribonucleic acid (RNA) in both fluids has been found up to 29 days after the onset of infection. Culture of Zika virus in urine and saliva has also been reported, with the virus cultured at day six after symptom onset for both fluids (9).
Reproductive counseling for women who desire pregnancy: Both women who are diagnosed with Zika virus disease and asymptomatic women with possible exposure to Zika virus should wait at least 8 weeks from symptom onset or exposure to attempt pregnancy, and their male partners diagnosed with Zika virus disease should wait at least 6 months from symptom onset to attempt pregnancy. Men who have possible Zika virus exposure without clinical illness consistent with Zika virus disease should wait at least 8 weeks after possible exposure before attempting pregnancy. This advice means that those living in areas with ongoing transmission of Zika virus may decide to delay pregnancy. Those who are not planning such delay should talk with their health care providers. Obstetrician-gynecologists and other health care providers should counsel patients on the risks of Zika as part of their pregnancy planning and counseling. Routine Zika virus testing is not currently recommended for women or men with possible Zika virus exposure without clinical illness who are attempting pregnancy. For men, this advice in part reflects uncertainty on how serologic testing detects the presence or absence of Zika virus in semen. No instances of Zika virus transmission during fertility treatment have been documented, but transmission through donated gametes or embryos is theoretically possible, given that Zika virus can be present in semen and sexual transmission of Zika virus has occurred.
Through blood transfusion
To date, there have not been any confirmed blood transfusion transmission cases in the United States. There have been multiple reports of blood transfusion transmission cases in Brazil. These reports are currently being investigated. During the French Polynesian outbreak, 2.8% of blood donors tested positive for Zika and in previous outbreaks, the virus has been found in blood donors. Zika Virus Blood Screening: Blood donor screening on the basis of a questionnaire, without a laboratory test, is insufficient for identifying Zika-infected donors in areas with active mosquito-borne transmission of Zika virus due to the high rate of asymptomatic infection. Although there is no FDA-licensed test for Zika virus, testing for Zika became available through two separate Investigational New Drug (IND) applications for blood collected in Puerto Rico and mainland United States. The tests became available on 3 April 2016 (Roche Molecular Systems, Inc.) and 20 June 2016 (Hologic, Inc. /Grifols) (8).
Through laboratory exposure
Prior to the current outbreak, there were four reports of laboratory acquired Zika virus infections, although the route of transmission was not clearly established in all cases. As of 15 June 2016, there has been one reported case of laboratory-acquired Zika virus disease in the United State (8).
Zika Virus Infection Confirmation

Currently, real-time reverse transcription polymerase chain reaction (rRT-PCR), immunoglobulin M (IgM), and plaque reduction neutralization (PRNT) tests are available to detect Zika infection, although each test has limitations. If a patient has had symptoms of Zika virus infection for less than 5 days, serum and urine should be obtained for rRT-PCR testing. If symptoms have been present for 5 to 14 days, urine should be tested by rRT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If rRT-PCR is performed within the appropriate period and the result is negative, Zika virus infection is excluded; if the result is positive, acute Zika virus infection is confirmed, and additional testing is not indicated; rRT-PCR can be performed by two commercial laboratories (Quest Diagnostic and LabCorp), state health departments, and the CDC.
If serum and urine is collected more than 5 days after symptoms onset and rRT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay of Zika virus. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plague reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected GBS, rRT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, rRT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue. Hill’s criteria to assess the evidence for causation is based on 9-factors. It states the criteria for establishing a link between microorganism and disease (11): Strength of association; Consistency; Specificity; Temporality; Biological gradient; Plausibility; Coherence; Experimental animal model; and Analogy. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard (12).
Zika Infection and Fetal Anomalies
Concurrent with the increasing rate of Zika infection in Brazil was an increase in the rate of microcephaly in the same regions; indeed, the prevalence of congenital microcephaly increased by a factor of twenty (10). In 2016, Rasmussen and colleagues found that the critical components of these criteria (stated above) are fulfilled and concluded that there is little doubt Zika virus is a proven and extremely dangerous teratogen, and they also concluded that the necessary 7 of these 9 Hill’s criteria have been met (13). Given their assessment of Shephard’s criteria, the authors argued that the link between maternal Zika virus infection and severe congenital anomalies has risen from association to well-defined causation. Some authors estimate the risk of microcephaly among infants of mothers infected with Zika to be between 0.9% and 13% (14). While this estimate is concerning, it must be considered with caution as it is based on modeling and limited retrospective data. Likewise, microcephaly is only one of several potentially adverse outcomes. It is well established that infants with severe microcephaly from other causes will experience a range of neurologic sequelae, but it is unclear if this is true for all cases of Zika-related microcephaly. Neurologic problems can range from intellectual disability to sensory deficient to seizures; they can be mild, severe, or life-threatening. Long-term outcomes are not at all clear and will not be for years to come.
Testing algorithm for a pregnant woman with possible Zika virus exposure NOT residing in an area with active Zika virus transmission:
Pregnant woman with history of travel to an area with ongoing Zika virus transmission should be tested for Zika virus infection. Current guidelines are (15):
I. If tested positive or conclusive for Zika virus infection: consider serial fetal ultrasound and consider amniocentesis for Zika virus testing.
II. If tested negative for Zika virus infection: fetal ultrasound to detect microcephaly or intracranial calcification;
- If microcephaly or intracranial calcifications are present: retest pregnant woman for Zika virus infection and consider amniocentesis for Zika virus testing.
- If microcephaly or intracranial calcifications are not present: routine prenatal care.
Testing algorithm for pregnant women residing in an area with active Zika virus transmission, with or without clinical illness consistent with Zika virus disease:
For pregnant woman residing in an area with ongoing Zika virus transmission, current guidelines are (15):
I. Pregnant woman reports clinical illness consistent with Zika virus disease.
- If testing is positive – Consider serial fetal ultrasounds and consider amniocentesis for Zika virus testing.
- If testing is negative – Fetal ultrasound to detect microcephaly or intracranial calcification. If microcephaly or intracranial calcifications are present – retest pregnant woman for Zika virus infection and consider amniocentesis for Zika virus testing. If microcephaly or intracranial calcifications are not present – routine prenatal care, retesting for Zika virus infection in mid-second trimester are recommended; and consider an additional fetal ultrasound.
II. Pregnant woman does not have clinical illness consistent with Zika virus disease; test for Zika virus infection upon initiation of prenatal care.
- If testing is positive or inconclusive test for Zika virus infection – Consider serial fetal ultrasounds and consider amniocentesis for Zika virus testing.
- If testing is negative for Zika virus infection – advised fetal ultrasound at 18-20 weeks of gestation and test for Zika virus infection in mid-second trimester. If microcephaly or intracranial calcifications are present, or positive or inconclusive test for Zika virus infection – consider serial fetal ultrasounds and consider amniocentesis for Zika virus testing. If microcephaly and intracranial calcifications are not present and negative test for Zika virus infection – routine prenatal care is advised and consider an additional ultrasound. At any time during pregnancy fetal microcephaly or intracranial calcifications are detected – consider retesting pregnant woman for Zika virus infection and consider amniocentesis.
In summary interpretation of laboratory results and prenatal management are:
- Recent Zika virus infection or recent flavivirus infection specific virus cannot be identified – Consider serial ultrasounds every 3-4 weeks to assess fetal anatomy and growth. Decision regarding amniocentesis should be individualized for each clinical circumstance.
- Presumptive recent Zika virus infection and presumptive recent flavivirus – Consider serial ultrasounds every 3-4 weeks to assess fetal anatomy and growth. Amniocentesis might be considered; decision should be individualized for each clinical circumstance.
- Recent dengue virus infection – Clinical management in accordance with existing guidelines.
- No evidence of Zika virus or dengue virus infection – A prenatal ultrasound to evaluate for fetal abnormalities consistent with congenital Zika virus syndrome. If fetal abnormalities are present: repeat Zika virus rRT-PCR and IgM test; base clinical management on corresponding laboratory results. If fetal abnormalities are absent: base obstetric care on the ongoing risk for Zika virus exposure risk to the pregnant woman.
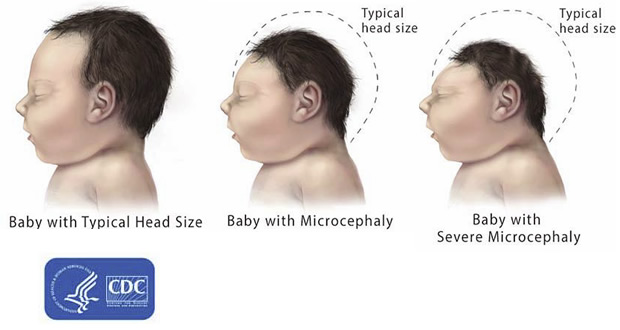
- Microcephaly is a condition where a baby is born with a small head or the head stops growing after birth;
- Microcephaly is a rare condition. One baby in several thousand is born with microcephaly;
- The most reliable way to assess whether a baby has microcephaly is to measure head circumference 24 hours after birth, compare the value with WHO growth standards, and continue to measure the rate of head growth in early infancy;
- Babies born with microcephaly may develop convulsions and suffer physical and learning disabilities as they grow older;
- There are no specific tests to determine if a baby will be born with microcephaly, but ultrasound scans in the third trimester of pregnancy can sometimes identify the problem;
- There is no specific treatment for microcephaly.
- A deoxyribonucleic acid (DNA)-based vaccine that uses a strategy similar to an investigational flavivirus vaccine for West Nile virus infection. That vaccine, which was developed by scientists at the National Institutes of Health- National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center, was found to be safe and induced an immune response when tested in a Phase 1 clinical trial. A DNA-based Zika vaccine candidate entered an early-stage trial at NIAID in August, 2016.
- A live-attenuated (in which the virus has been weakened so that it cannot cause disease) investigational Zika vaccine building on a similar vaccine approach for the closely related dengue virus. The dengue vaccine candidate was shown to be safe and immunogenic in early-phase trials, and is currently being evaluated in a large Phase III study in Brazil.
- An investigational Zika vaccine that uses a genetically engineered version of vesicular stomatitis virus – an animal virus that primarily affects cattle. Vesicular stomatitis virus (VSV) was successfully used in an investigational Ebola vaccine tested by NIAID. This vaccine approach is at an early stage with plans underway to evaluate the Zika vaccine candidate in tissue culture and animal models.
- A whole-particle inactivated Zika vaccine based on a similar vaccine approach used by the Walter Reed Army Institute of Research (WRAIR) to develop vaccines against the related Japanese Encephalitis and dengue viruses.
- World Health Organization
Zika Virus and Safe Blood Supply: Question and Answer
http://www.who.int/features/qa/zika-safe-blood/en/ - Centers for Disease Control and Prevention (CDC)
Travel Notices and Advisory
http://wwwnc.cdc.gov/travel/notices/ - National Institutes of Health (NIH)
Zika Virus Vaccines
https://www.niaid.nih.gov/diseases-conditions/zika-vaccines - Petersen LR, Jamieson DJ, Powers AM, et al. Zika Virus. NEJM 2016;374(16):1552-1563
- World Health Organization. Zika Animated Infographic; https://youtu.be/ghxHxNAi_gI Last Accessed 24 August 2016
- Duffy MR, Chen T, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. NEJM; 2009;360(24):2536-2543
- Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ 2016;352:i657
- World Health Organization. Zika Situation Report. Available at http://www.who.int/topics/zika/en/ Accessed on 25 August 2016
- Mkakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. NEJM 2016;374(10):951-958
- Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. NEJM 2016;374(22):2142-2151
- Centers for Disease Control and Prevention. Zika Virus: Transmission & Risks. http://www.cdc.gov/zika/transmission/index.html Accessed 1September 2016
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis 2016;16:405-405
- Brasil P, Pereira JP, Gabaglia CR, et al. Zika virus infection in pregnant women in Rio de Janeiro-Preliminary report [published online March 4, 2016]. NEJM 2016
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. NEJM 2016:374(10):951-958
- Shepard TH. “Proof” of human teratogenicity. Teratology 1994;50(2):97-98
- Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika virus and birth defects – reviewing the evidence for causality. NEJM 2016;374(20):1981-1987
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, et al. Zika and the risk of microcephaly. NEJM 2016;375(1):1-4
- Schuler-Faccini L, Riberio EM, Feitosa IM, et al. Possible Association Between Zika Virus Infection and Microcephaly – Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:59-62
- Centers of Disease Control and Prevention. Collecting and submitting fetal tissue specimens for Zika virus testing. Available at http://www.cdc.gov/zika/hc-providers/test-specimens-at-time-of-birth.html Retrieved on 29 September 2016
- European Centre for Disease Prevention and Control. Zika virus disease epidemic potential association with microcephaly and Guillain-Barre syndrome (first update). http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/pages/index.aspx Accessed 2 October 2016
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016;387(10027):1531-1539
- Carteaux G, Maquart M, Badet A, et al. Zika virus associated with meningoencephalitis. NEJM 2016;374(16):1595-1596
- Mecharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet 2016;387(10026:1481
- National Institutes of Health; National Institute of Allergy and Infectious Diseases. Safety and Immunogenicity of a Zika Virus DNA Vaccine, VRC-ZKADNA085-00-VP, in Healthy Adults https://clinicaltrials.gov/ct2/show/NCT02840487 Accessed on 22 September 2016
- Soares AA. Promising new tools to fight Aedes mosquitoes. Bull World Health Organ 2016;94:562-563
Microcephaly
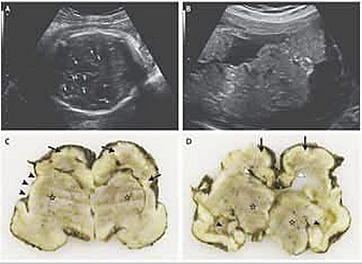
Microcephaly is a neonatal malformation defined as a head size much smaller compared with other babies of the same age and sex. If this combines with poor brain growth, babies with microcephaly can develop developmental disabilities. The severity of microcephaly ranges from mild to severe. It is characterized by a typical disproportion in size between the skull and the face. The forehead is sloping. The brain is small; the cerebral hemispheres are affected to a greater extent than the diencephalic and rhombencephalic structures. Abnormal convolutional patterns, including macrogyria, microgyria, and agyria, are frequently found. The ventricles may be enlarged. Many difficulties arise in attempting to identify fetal microcephaly. The utility of head measurement alone may be hampered by incorrect dating or intrauterine growth restriction. Furthermore, the natural history of fetal microcephaly is largely unknown. A progressive development of the lesion interfering with early recognition has been described. A comparison of biometric parameters such as head circumference – abdominal circumference ratio has been suggested. A qualitative evaluation of the intracranial structures is a useful adjunct to biometry because many cases on microcephaly are associated with morphologic derangement, particularly with ventriculomegaly, schizenecephaly, and disorders of ventral induction.
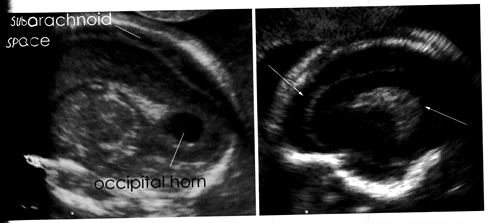
A nomogram of the normal dimensions of the frontal lobes has been developed and may prove useful in dubious cases. It has been suggested that vaginal sonography and color Doppler may be useful in the evaluation of fetuses with suspicious cranial measurements.
Key facts:
After the Baby is Born
To diagnose microcephaly after birth, a healthcare provider will measure the distance around a newborn baby’s head, also called the head circumference, during a physical exam. The provider then compares this measurement to population standards by sex and age. Microcephaly is defined as a head circumference measurement that is smaller than a certain value for babies of the same age and sex. This measurement value for microcephaly is usually less than 2 standard deviations (SDs) below the average. The measurement value also may be designated as less than the 3rd percentile. This means the baby’s head is extremely small compared to babies of the same age and sex.
Head circumference growth charts for newborns, infants, and children up to age 20 years in the United States can be found on CDC’s growth charts website, http://www.cdc.gov/growthcharts/clinical_charts.htm
Head circumference growth charts based on gestational age at birth (in other words, how far along the pregnancy was at the time of delivery) are also available from INTERGROWTH 21st, http://www.cdc.gov/zika/public-health-partners/microcephaly-case-definitions.html
CDC recommends that health care providers use the WHO growth charts to monitor growth for infants and children ages 0 to 2 years of age in the United States.
Often, healthcare providers should take the head circumference measurement when the newborn baby is at least 24 hours old. This helps make sure that compression due to delivery through the birth canal has resolved. If the healthcare provider suspects the baby has microcephaly, he or she can request one or more tests to help confirm the diagnosis. For example, special tests like a computed tomography (CT) scan or magnetic resonance imaging (MRI) can provide critical information on the structure of the baby’s brain that can help determine if the newborn baby had an infection during pregnancy. They also can help the healthcare provider look for other problems that might be present.
Postnatal Management
An important feature of the CDC guidance is the recommendation that specimens obtained after Zika virus or flavivirus infection is suspected or diagnosed should be sent to pathology for further according to CDC’s guidance on collecting and submitting fetal tissue specimens for Zika virus testing (16). For women with recent Zika virus or flavivirus infection, submitting placental and umbilical cord specimens for live births or submitting fetal tissue specimens for fetal loss is recommended for immunohistochemical (IHC) staining and rRT-PCR testing. For women with presumptive Zika virus or flavivirus infection, submitting placental and umbilical cord specimens for live births or submitting fetal tissue specimens for fetal loss should be considered for IHC staining and rRT-PCR testing.
For women with possible exposure but for whom maternal testing did not occur, or for whom testing was performed more than 12 weeks after exposure, maternal Zika virus testing should be performed and placental testing may also be considered. For those presenting outside the recommended testing window, providers may consider IgM antibody testing. If fetal abnormalities associated with Zika virus infection are present, rRT-PCR testing should also be performed on maternal serum and urine. Pregnant women with laboratory evidence of confirmed or possible Zika virus infection who experience a fetal loss or stillbirth should be offered fetal pathology testing for Zika virus infection; testing includes submitting fetal tissue specimens for rRT-PCR and IHC staining. Infant testing and management should follow CDC guidelines, noting that cord blood specimens should no longer be submitted (16).
Breastfeeding
Although the presence of Zika virus in breast milk has been reported, there are no reports of infants getting Zika virus through breast feeding. Infection through oral intake is not known. The benefits of breastfeeding likely outweigh the potential neonatal risk. Therefore, the recommendation is that women should continue to breastfeed, even in where Zika virus is found (16).
Future Pregnancies
Based on the available evidence, we think that Zika virus infection in a woman who is not pregnant would not pose a risk for birth defects in future pregnancies after the virus has cleared from her blood. From what we know about similar infections, once a person has been infected with Zika virus, he or she is likely to be protected from a future Zika infection.
Zika Virus Infection and Neurologic Complications
Zika virus infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the Zika virus outbreak (17). GBS is and acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted during the 2013-2014 outbreak in French Polynesia, the association between Zika virus infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with Zika virus infection but no neurologic complications (18). Symptoms of Zika virus infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The Zika virus IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patient had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 Zika virus infections (19). Other neurologic complications that have been associated with Zika virus infection are meningoencephalitis, brain ischemia, and myelitis (20).
Zika Virus Vaccines
There are multiple vaccines for Zika virus infection prevention in development. These include:
Early-stage trials examine whether an experimental vaccine is safe and generates immune responses in vaccinated volunteers. A safe and effective, fully licensed Zika vaccine will likely not be available for several years (21).
Promising New Tools to fight Aedes mosquitoes
Wolbachia-infected Aedes aegypti was one of five promising new tools to reduce mosquito populations discussed at an emergency meeting of the World Health Organization (WHO) Vector Control Advisory Group in March in Geneva. The meeting was held a month after WHO declared the Zika epidemic an international public health emergency. At that meeting, experts from the Vector Control Advisory Group reviewed four other new tools: transgenic mosquitoes called Oxitec OX513A, vector traps, the sterile insect technique and the attractive toxic sugar bait. They recommended the pilot deployment of two contrasting approaches: Wolbachia-infected Aedes aegypti mosquitoes and Oxitec transgenic mosquitoes to see whether the latter reduces mosquito populations when released on a large scale. While the Wolbachia approach aims to make Aedes mosquito populations less harmful to human health, the Oxitec approach seeks to reduce the size of these populations. Scientists at Oxitec, a subsidiary of the biotechnology company Intrexon based in England, developed the genetically modified mosquitoes back in 2002. These mosquitoes have a gene that prevents their offspring from surviving to maturity. Only males are released, so there is no effect on disease transmission because only females bite humans. Once free, they mate with wild females and pass this self-limiting gene on to future generations. Oxitec is awaiting approval from the United States Food and Drug Administration to do a large-scale trial near Key West in the southern state of Florida. The company has been deploying genetically modified mosquitoes in Piracicaba in the Brazilian state of São Paulo in collaboration with the city authorities since April last year (22).
Summary
Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly. The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy. Zika virus has now been established as the cause of Guillain-Barré syndrome (GBS) in adults. Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented. Diagnosis in adults made with symptoms of less than 5 days duration, the best tests to confirm Zika virus infection are serum and urine rRT-PCR test and should be performed when suspected. If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M (IgM) assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus. Zika virus infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
Zika virus infection in humans appears to have changed in character while expanding in geographical range. The change is from an endemic arbovirus causing mild illness across equatorial Africa and Asia. From 2007 onwards, Zika virus caused large outbreaks in previously unexposed populations, and from 2013 onwards, outbreaks linked with neurological disorders including Guillain-Barre syndrome and congenital malformations, for reasons that are not yet known. The future transmission of Zika infection is likely to coincide with the global distribution of Aedes vectors. Person-to-person transmission, both vertically, from mother to fetus, and horizontally through sexual transmission, is also expected to continue, and we anticipate that infections will be carried widely by international travel.
Suggested Reading
References
Опубликован: 8 May 2020
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


