Seizure Disorders and Pregnancy
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Roughly one out of every 100 pregnancies occurs in a woman with epilepsy. These pregnancies present a unique challenge to obstetricians and neurologists due to the interrelationship of the effects of epilepsy and pregnancy, the variable effects of anti-convulsant medications on mother and fetus, and the changes in pharmacokinetics of these medications during pregnancy. The obstetricians and neurologist should work together prior to conception and throughout the patient's pregnancy to determine the safest and most effective medical therapy. Furthermore, the pediatrician selected by the patient to care for her baby should be included in pre-pregnancy discussions to address the potential increase in congenital malformations, the potential for neonatal sedation with certain medications, and questions concerning breast-feeding. The purpose of this document is to provide the current information on this issue and to offer practical advice on managing patients.
Epilepsy: The Clinical Problem
Epilepsy, which is defined as two or more seizures that are not provoked by other illnesses or circumstances, affects about 45 million people worldwide. In the United States, the prevalence of epilepsy is approximately 6 to 8 per 1000 population, and the incidence is approximately 26 to 40 per 100,000 person-years, with higher rates among infants and persons older than 60 years of age. Approximately 70% of adults with new-onset epilepsy have partial (focal) seizures. In the majority of cases (62%), the cause is unknown. Stroke (9.0%), head trauma (9.0%), alcohol (6.0%), neurodegenerative disease (4.0%), static encephalopathy (3.5%), brain tumor (3.0%) and infection (2.0%) account for most remaining cases (5). Although cerebrovascular causes are more common in the elderly, the cause is still unknown in 25 to 40% of patients who are 65 years of age or older. The transient occurrence of altered awareness, abnormal behavior, or involuntary movements suggests a diagnosis of epilepsy. Because epileptic seizures are rarely observed by a physician, the diagnosis is typically based on historical information supplemented by selected tests. The first step is to answer the question of whether the event was a seizure. The second is to determine whether the patient has epilepsy.
The neurologic examination is normal in most patients with epilepsy. According to joint recommendations of the American Academy of Neurology and the American Epilepsy Society, patients with an unprovoked first seizure should undergo electroencephalography (EEG), computed tomographic (CT) scanning or magnetic resonance imaging (MRI) of the head, and selected blood tests according to clinical circumstances (3). Epileptiform EEG patterns such as spikes and sharp waves can assist in the diagnosis and in classifying seizures as being either focal or generalized. However, neither a normal EEG nor interictal abnormalities alone refute or confirm a diagnosis of epilepsy. EEGs are abnormal in 50% of patients representing with a first seizure, and they show epileptiform discharges in only about half of these patients. The incidence of abnormalities increases when EEGs are repeated or performed after the patient has undergone sleep deprivation (7). Video EEG monitoring is necessary if there is concern about non-epileptic events. MRI of brain is more sensitive than CT in identifying structural lesions causally related to epilepsy. Routine blood tests rarely inform the diagnosis in otherwise healthy patients. However, a complete blood count, liver-function tests, and measurement of electrolyte levels are useful before antiepileptic treatment is initiated, since dosage adjustment may be necessary if hepatic or renal function is abnormal. A diagnosis of epilepsy can have a considerable effect on the patient's mood, interpersonal relationships, employability, social functioning, quality of life, and ability to drive. Early and repeated discussions of these issues are suggested.
Pre-Pregnancy Counseling
Women with seizure disorders should seek care from an obstetrician-gynecologist as soon as they become sexually active. The use of certain anti-epileptic medications may interfere with the action of oral contraceptive agents. Patients taking low-dose oral contraceptives and certain antiepileptic medications may have more breakthrough bleeding and may be at increased risk for unplanned pregnancy. This rapid clearance does not appear to be induced by use of valproate or benzodiazepines (1). Although fertility rates may be lower in patients with epilepsy, most patients with epilepsy are able to conceive without difficulty.
Topics to address in pre-pregnancy counseling of women with epilepsy are the possibility that seizures will become more frequent during pregnancy and teratogenic potential of antiepileptic drugs. Importance of good seizure control and adherence to the antiepileptic drug regimen should be stressed upon. Monitor of plasma levels of anti-epileptic drugs should be given to the patients. Pre-conceptional and gestational folate supplementation and vitamin K supplementation are important part of counseling.
Effects of Pregnancy on Epilepsy
For some women with epilepsy, changes in the frequency of seizures will occur throughout pregnancy, but the time of greatest risk for seizures is during labor and delivery. In about 48% to 57% of women with seizures disorders the frequency of seizures during pregnancy remains stable. Increase is seen in one quarter to one third, and actually decreases in 9% to 22% (2). Patients with seizure disorders may be treated with single medications or a combination of medicines, based on the type of seizure and side effects. Serum levels of the most frequently used anticonvulsant medications (phenytoin, carbamazepine, and phenobarbitol) can change dramatically during pregnancy, generally decreasing in total concentration as pregnancy progresses. Although total levels fall, the free (active) levels tend to decrease because of a decline in serum albumin and other proteins throughout pregnancy. The drug dosage should be adjusted according to the total serum level and the patient's condition. If drug levels are carefully monitored, pregnancy should have little effect on seizure frequency.
Potential toxic effects of commonly used anticonvulsants:
Source: ACOG Educational Bulletin; Number 231.
| Medication | Maternal Effects | Characteristic Potential Fetal/Neonatal Effects |
|---|---|---|
| Carbamazepine Oxcarbazepine Levetiracetam | Drowsiness, leukopenia,Ataxia, mild heptotoxicity | Facial dysmorphisms, neural tube defects,Hypoplasia of distal phalanges |
| Phenobarbital | Drowsiness, ataxia | Neonatal withdrawal, neonatal coagulopathy |
| Phenytoin Gabapentin | Nystagmus, ataxia, hirsutism,Gingival hyperplasia, megaloblastic anemia | Facial clefting, hypoplasia of distal phalanges, hypertelorism, neonatal coagulopathy |
| Primidone Tiagabine | Drowsiness, ataxia, nausea | Neonatal withdrawal, neonatal coagulopathy |
| Valproic acid | Ataxia, drowsiness, alopecia,Hepatotoxicity, thrombocytopenia | Facial dysmorphisms, neural tube defects |
Effects of Epilepsy on Pregnancy
Most women with seizure disorders who become pregnant will have an uneventful pregnancy with an excellent outcome. Several complications have been reported; these are: preeclampsia, still-birth, depressed Apgar scores, low birth-weight, diminished head circumference. However, studies are reassuring, that, there is no increase in the rates of perinatal mortality, preeclampsia, preterm labor, or cesarean delivery in women with epilepsy.
Fetal and Neonatal Effects
Vitamin Deficiencies: all anticonvulsants interfere with folic acid metabolism. Folic acid deficiency during embryogenesis has been associated with neural tube defects and other congenital malformations. Pre-conceptional and during pregnancy the folic acid dose of 4 mg per day is appropriate for the patients taking anticonvulsants. Neonatal hemorrhage due to decreased vitamin K dependent clotting factors (II, VII, IX, X) has occurred in infants born to mothers taking phenobarbital, phenytoin, and primidone. It is recommended these infants be given 1 mg of vitamin K intramuscularly at birth. Some authors recommend prophylactic oral vitamin K during last month of pregnancy. Therapy with phenobarbital, phenytoin and primidone may also result in increased metabolism of vitamin D. Patients should be encouraged to take prenatal vitamins that include an adequate amount of vitamin D.
Congenital Malformations: in literature it appears to be a 6-8% chance of birth defects in infants born to women taking anticonvulsant medications. This represents a risk two to three times that of the general population. Predominate malformations are cleft lip/palate and cardiovascular malformations. A specific fetal hydantoin syndrome has been identified consisting of growth and performance delays, cranial facial abnormalities (including clefting), and limb abnormalities (including hypoplasia of nails and distal phalanges). Approximately 10-30% of infants born to women taking these drugs have been reported to have some aspects of the syndrome (3). The proportion of exposed infants having the complete syndrome is much smaller. Teenagers with seizure disorders are often treated with valproate because it has few side-effects in this age group. It has, however, been associated with specific fetal valproate syndrome of cranial-facial defects and neural tube defects. Researchers have found a statistically significant difference in IQ among children of mothers with seizure disorders compared with controls. There appears to be a small, undefined risk of a slightly decreased IQ in children born to mothers with epilepsy.
n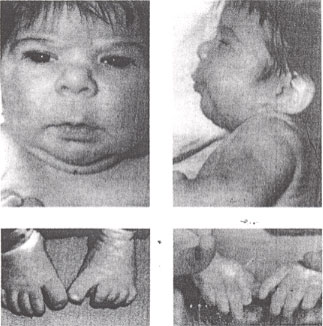
Fetal hydantoin syndrome: facial features - upturned nose, mild midfacial hypoplasia, long upper lip with thin vermilion border and lower distal digital hypoplasia.
Possible mechanisms by which anti-epileptics may cause birth defects are:
- The conversion of antiepileptic drugs in the embryo into highly unstable toxic metabolites (bio-activation process) may damage fetal DNA.
- Anti-epileptics may alter the endogenous concentrations of retinoids (which modulate embryonic growth, differentiation, and morphogenesis).
- Anti-epileptics like phenobarbital and phenytoin can cause folic acid deficiency by impairing its absorption, metabolism, or both. Deficiencies of folic acid can raise homocysteine levels (which are thought to underlie neural tube defects).
Management
During Pregnancy: The patients with anti-convulsant level should be monitored periodically and dosages adjusted accordingly. Good seizure control during pregnancy is important in ensuring the well-being of the woman with epilepsy and that of the fetus. If at all possible, maintain a pregnant patient with epilepsy on mono-therapy at the lowest effective dose, which ideally should be established before conception. Monitor anti-epileptic drug levels to ensure the therapeutic drug levels. Lamotrigine may require a two-fold increase by the third trimester in order to maintain stable serum concentrations, but metabolism returns to the pre-pregnancy baseline within 48 hours after delivery, necessitating a rapid postpartum reduction in dosage. Patients taking anticonvulsants should be evaluated for possible fetal neural tube defects with a combination of maternal serum alpha-fetoprotein determination and ultrasound. Amniocentesis should be offered if preceding tests are equivocal. At 16-18 weeks of gestation, the patients should undergo a comprehensive ultrasound examination to look for congenital malformations. Antepartum fetal surveillance tests should be done for obstetric indications. Monitoring and controlling risk factors like sleep deprivation or non-adherence that may provoke more frequent seizures during pregnancy are helpful in good outcome. If a woman is adhering to the therapeutic regimen and her risk factors are well controlled, but seizures increase anyway, consider increasing the dosage of her anticonvulsants.
Labor and Delivery: With appropriate fetal monitoring, the availability of good obstetric anesthesia, the ability to measure maternal anticonvulsant levels, vaginal delivery can be accomplished safely in the patient with a seizure disorder. The delivery plan should include the availability of personnel to perform neonatal resuscitation if necessary. During labor, oral absorption of medications is erratic; if the patient vomits, it is almost negligible. When administration of anticonvulsant is necessary during labor, levels should be determined to help ascertain the appropriate dosage. If the patient's phenytoin level is normal, the usual daily dose may be administered intravenously. In patients with therapeutic serum levels of phenobarbital, a single 60-90 mg intramuscular dose will usually be sufficient for maintenance throughout labor and delivery. The usual loading dosage is 10-15 mg/kg administered intravenously at a rate no faster than 50 mg/min. Patients taking carbamazepine can be given intravenously phenytoin because there is no parenteral form. Benzodiazepines also may be used for acute seizures, but they can cause early neonatal depression as well as maternal apnea.
Occasionally seizures will be diagnosed for the first time during pregnancy. If the seizures occur in the third trimester, they may be confused with eclampsia. The diagnosis often becomes clearer over time, but in either case action must be undertaken to prevent additional seizures. In these cases, it is best to assume the patient has eclampsia. If patient has recurrent generalized seizures (status epileptics), immediate treatment is essential. Consultation with an anesthesiologist and neurologist may be helpful. The drug of choice is intravenous phenytoin, which is highly effective, has long duration of action, and has a low incidence of serious side-effects. Alternatively, phenobarbital or diazepam may be used. Status epilepticus may lead to maternal fetal hypoxemia. The patient should be placed on her left side if possible as this will increase uterine blood flow as well as decrease the risk of maternal aspiration. Oxygen should also be administered if possible. Risk of placental abruption also exists with prolong seizures.
Breastfeeding: Most health organizations strongly recommend breastfeeding to promote mother-child bonding and reduce the risk of infection and immunological disorders in later life (4). Antiepileptic drugs cross into breast milk in varying degrees, usually through simple diffusion, and the ratio is determined by the drug's molecular weight, pKa, lipophilicity, and most importantly the degree of protein binding. Concentrations in breast milk of phenytoin, carbamazepine, valproate, and tiagabine are negligible because they bind tightly to proteins. The best advice for most women is to seriously consider breastfeeding, keeping in mind that once started, the infant can be observed for proper weight gain and sleep cycles. Anticonvulsant metabolism and clearance remains elevated as long as patient continues to breastfeed. When patient stops breastfeeding, the mother may experience an increase in serum anticonvulsant drugs concentrations requiring a dosage adjustment. If breastfeeding is suddenly stopped, some infants who had been exposed to these medications may experience withdrawal symptoms. These usually occur in the first few days after breastfeeding is stopped. Infants may need to be started on low-dose phenobarbital and undergo gradual withdrawal.
Postpartum Period: the levels of anticonvulsant medications may rise rapidly during the first few weeks postpartum and should be monitored frequently. One approach is measure the serum level approximately 1 week postpartum to guide dosage adjustments. Women should be counseled about contraception. No method is contraindicated for patents with epilepsy, although if oral contraceptives are selected higher doses may be required. The patient should be encouraged to continue to receive care to control her condition and receive pre-conceptional care for a future anticipated pregnancy.
Choice of Antiepileptic Drugs in Women
Antiepileptic drugs, and especially valproate, have been associated with reproductive endocrine disorders, most notably features of polycystic ovary syndrome (e.g. irregular menstrual cycles, weight gain, and hirsutism) (6). This association appears to be related at least in part to the epilepsy itself, but in majority of women, medication seems to play the major role. Observational studies have shown clinically important associations between the use of valproate, alone or in combination with other drugs, and the development of polycystic ovaries, anovulatory cycles, and hyperandrogenism (8). Hepatic enzyme-inducing antiepileptic drugs such as phenytoin, carbamazepine, and phenobarbital, as well as topiramate and oxcarbazepine, increase the clearance of oral contraceptive pills. Thus, women taking these drugs who use oral contraceptive pills are advised to use preparations containing at least 50 µg of ethinyl estradiol in order to reduce the chance of pregnancy (9). However, the contraceptive efficacy of higher-dose oral contraceptive pills has not been well studied, and alternative methods (e.g. barrier contraception) should be discussed. The dosage of lamotrigine requires adjustment when oral contraceptive pills are started or discontinued, because oral contraceptives enhance the clearance of lamotrigine. Serum concentrations of lamotrigine should be followed in this setting and in pregnancy (9), which increases the clearance of many antiepileptic drugs, but particularly that of lamotrigine.
Babies born to women with epilepsy have an increased rate of malformations; this is believed to be attributable mostly to antiepileptic drugs (10). Studies of the effect of specific drugs during pregnancy are hampered by confounding factors such as the type and severity of epilepsy and the use of more than one agent in many patients. No antiepileptic drug can be considered to be absolutely safe. Newer drugs are less well studied, but the evidence linking valproate to an increased risk of birth defects is most convincing and sufficient to advice against its use in women of childbearing age unless there is no alternative (11). The risk of birth defects is likely to be minimized further by treating with monotherapy and drug dosages as low as possible during pregnancy, although the evidence to support these recommendations is limited. Retrospective analyses of school-age children have suggested associations between intrauterine exposure to valproate (but not other antiepileptic drugs) and lower IQ scores and development delay (12); this finding warrants confirmation in prospective studies.
Lamotrigine (Lamictal) is used for epilepsy and bipolar disorders. There are no adequate and well controlled studies in pregnancy. Patients may experience increased risk of seizures in absence of level monitoring (13). It crosses placenta and fetal exposure has not been documented to increase the risk of major anomalies. Its transfer to breast milk is low and it is considered safe for breastfeeding.
Summary
Epilepsy posed unique challenges for both a pregnant woman and her clinician. Physiologic changes during pregnancy can influence epilepsy and conversely epilepsy and anticonvulsant medications can affect pre-pregnancy and its outcomes. Medical advances continue to help minimize fetal and maternal risk and have reduced the number of major birth defects and miscarriages among women with epilepsy. With continued vigilance among pregnant women with epilepsy and their healthcare providers, fetal and maternal outcomes will continue to improve.
References
- Battino D, Tomson T. Management of epilepsy during pregnancy. Drugs 2007;67:2727-2746
- McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet 2002;41:559-579
- Krumholz A, Wiebe S, Gronseth G et al. Evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007;69:1996-2007
- Kjaer D, Horvath-Puho E, Christensen J et al. Antiepileptic drug use, folic acid supplementation, and congenital abnormalities: a population-based case-control study. BJOG 2008;115:98-103
- Banerjee PN, Hauser WA. Incidence and prevalence. In: Engel J Jr, Pedley TA, eds. Epilepsy: a comprehensive textbook. 2nd ed. Baltimore: Wolters Kluwer/Lippincott Williams & Wilkins. 2008;45-56
- Herzog AG, Schachter SC. Valproate and the polycystic ovarian syndrome: final thoughts. Epilepsia 2001;42:311-315)
- Fisch B, So E. Activation methods. In: Ebersole JS, Pedley TA, eds. Current practice of clinical electroencephalography. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2003:262-264
- Harden CL. Polycystic ovaries and polycystic ovary syndrome in epilepsy: evidence for neurogonadal disease. Epilepsy Curr 2005;5:142-146
- Pennell PB, Gidal BE, Sabers A et al. Pharmacology of antiepileptic drugs during pregnancy and lactation. Epilepsy Behav 2007;11:263-269
- Barrett C, Richens A. Epilepsy and pregnancy: report of an Epilepsy Research Foundation workshop. Epilepsy Res 2003;52:147-187
- Duncan S. Teratogenesis of sodium valproate. Curr Opin Neurol 2007;20:175-180
- French JA, Pedley TA. Initial management of epilepsy. N Engl J Med 2008;359:166-176
- Buhimschi CS, Weiner CP. Medications in pregnancy and lactation. Obstet Gynecol 2009;113:166-188
Published: 8 June 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


