Birth Trauma: Neonatal Brachial Plexus Injury
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
The feared consequence of shoulder dystocia is permanent obstetrical brachial plexus injury and/or fetal neurologic damage caused by reduced cord blood flow and fetal asphyxia. Occasionally, shoulder dystocia results in fetal death. Suspected macrosomia is encountered commonly in obstetrical practice. As birth weight increases, the likelihood of labor abnormalities, shoulder dystocia, birth trauma, and permanent injury to the newborn increases. Two terms are applied to excessive fetal growth: "large for gestational age" (LGA) and "macrosomia." Shoulder dystocia, which affects both mother and fetus, is a problem involving the impaction of the fetal shoulder behind the maternal symphysis pubis. Shoulder dystocia is a "bony problem." Although shoulder dystocia cannot be predicted reliably, there are multiple risk factors. The primary objective in the presence of clinically recognizable shoulder dystocia continues to be the delivery of fetus before the fetal brain experiences hypoxic-ischemic injury.
The purpose of this document to quantify risks, address the accuracy and limitations of estimating fetal weight and suggest clinical management of a pregnancy with suspected macrosomia. The review emphasizes on neonatal brachial plexus palsy (NBPP) with special focus on its pathophysiology and causation. Some strategies that demonstrate either a reduction in NBPP or an increased rate of successful resolution of shoulder dystocia are included. The review also addresses the ways to reduce the incidence and reduce the risk of litigation.
Frequency of Macrosomia and Neonatal Brachial Plexus Palsy (NBPP)
Data from the National Center for Health Statistics show that 7.8% of all live-born newborns in the United States weigh 4,000 g or more (1). Only 1% weigh more than 4,500 g and 0.1% more than 5,000 g (1). Women with gestational diabetes mellitus or obesity have higher rates of LGA newborns and fetal morbidity and mortality. Macrosomia increases the risk of shoulder dystocia. Shoulder dystocia occurs in 0.2 - 3.0% of all vaginal births and the risk increases to 9 - 14% when birth weight is more than 4,500 g (2).
Clinically, neonatal brachial plexus palsy (NBPP) presents in a newborn as a weak or paralyzed upper extremity, with the passive range of motion greater than the active. The overall incidence of NBPP, both transient and persistent impairment, is 1.5 per 1,000 total births (3). Multiple reports in the peer-reviewed literature describe the occurrence of NBPP without concomitant clinically recognizable shoulder dystocia at the time of both vaginal and cesarean delivery. 10% to 30% of these injuries persist for years following birth.
The term "macrosomia" implies growth beyond an absolute birth weight, historically 4,000 g or 4,500 g, regardless of the gestational age, although establishing a universally accepted definition for macrosomia is challenging. Many authors and clinicians divide macrosomia into three categories, each with differing types and levels of risk: 1) 4.000 - 4,499 g, 2) 4,500 - 4,999 g and 3) more than 5,000 g.
Risk Factors
Various risk factors have been described in association with NBPP. They include fetal malposition, labor induction, labor abnormalities, operative vaginal delivery, fetal macrosomia, and shoulder dystocia. Overall, except for shoulder dystocia, these risk factors have not been shown to be statistically significant or clinically useful predictors for the occurrence of NBPP. Most cases of NBPP (greater than 80%) occur in women without known risk factors (4). Risk factors for shoulder dystocia are not reliable predictors for its occurrence or the occurrence of NBPP. Thus, no intervention has been identified that will prevent all or even most cases of NBPP or clinically apparent shoulder dystocia.
Notwithstanding the unreliability of specific risk-factors to predict NBPP or clinically apparent shoulder dystocia in a specific case, there are three clinical situations of concern to practitioners in which an alteration of usual obstetric management might be considered to reduce the risk of shoulder dystocia and NBPP:
- Suspected fetal macrosomia with estimated fetal weight exceeding 5,000 g in women without diabetes or 4,500 g in women with diabetes;
- Prior recognized shoulder dystocia, especially with a severe neonatal injury; and
- Mid-pelvic operative vaginal with fetal birth weight more than 4,000 g.
Even in these circumstances, the occurrence of NBPP is relatively low, and with proper informed consent, numerous clinical situations exist in which these risk factors alone should not dictate a particular course of management.
Pathophysiology of Neonatal Brachial Plexus Palsy (NBPP)
Uterine contractions and maternal pushing efforts produce pressure within the uterus that is transmitted by direct contact and through amniotic fluid. This pressure produces a force that moves the fetus into and through the pelvis. Both maternal (endogenous) forces and clinician-applied (exogenous) forces have a direct effect on the fetus as a whole and on its discrete anatomic structures (5). Maternal forces alone are an accepted cause of at least transient NBPP by most investigators. In addition, NBPP can be caused by downward lateral traction applied by the birth attendant. Downward lateral traction (bending of the fetus' neck away from the anterior shoulder, toward the posterior shoulder or floor) has been shown to place greater strain on the brachial plexus than downward axial traction, wherein the applied forces are parallel to the fetus cervicothoracic spine (3). Nonetheless, even properly applied axial traction can result in NBPP.
Neither high-quality nor consistent data exist to suggest that NBPP can be caused only by a specific amount of applied force beyond that typically used by healthcare providers during any delivery. Instead, available data suggest that the occurrence of NBPP is a complex event, dependent not only on the forces applied at the moment of delivery, but also on the constellation of forces (e.g., vector and rate of application) that have been acting on the fetus during the labor and delivery process, as well as individual fetal tissue characteristics (e.g., in situ strain and acid-base balance). In addition to research within the obstetric community, the pediatric, orthopedic, and neurologic literature now stress that, the existence of NBPP following birth, does not give prior indication that exogenous forces are the cause of this injury.
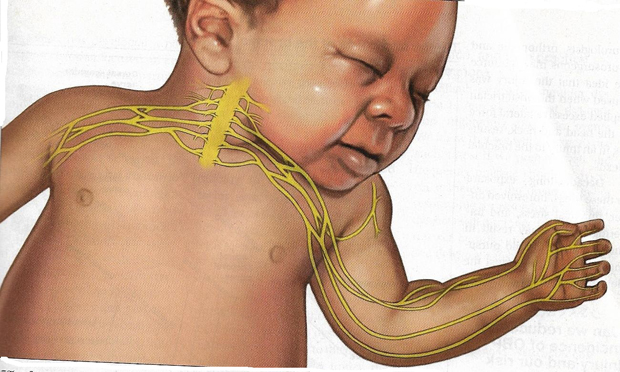
Figure 1. The Brachial Plexus Nerves. The forces of labor, fetal position, maternal pushing, and forces applied to the fetal head and neck by a birth attendant all may contribute to NBPP.
Types of Obstetric Brachial Plexus Injuries
Erb's Palsy: Injuries to the C5 and C6 nerves account for about 50% of cases. The muscle groups impacted by this injury include the deltoid and infraspinatus muscles (mainly C5) and the biceps muscle (mainly C6). This pattern results in an adducted and internally rotated upper arm, an extended forearm, with preservation of hand and wrist movement.
Erb's Palsy Plus: Injury to C5, C6, and C7 nerves accounts for about 35% of cases and manifests as adduction and internal rotation of the arm, extension, and pronation of the forearm, plus flexion of the wrist and fingers - the so-called waiter's tip posture.
Klumpke's Palsy: Isolated injury to the C8 and T1 root occurs infrequently and manifests as isolated hand paralysis and Horner's syndrome https://rarediseases.info.nih.gov/diseases/6670/horners-syndrome.
Approximately 1 in 5 NBPP injuries persist for years following birth. The incidence of NBPP ranges from 1.0 to 3.0 cases per 1,000 births (6). In a review of studies with at least 3 years of follow up and less than 10% loss to follow up, the authors reported that the three best observed a risk of persistent NBPP of 10%, 19% and 27% (7).
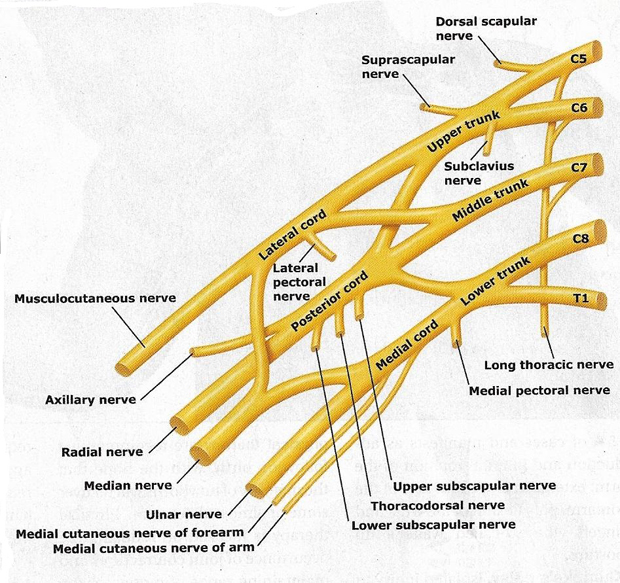
Figure 2. Trunks and cords of the brachial plexus. C5 and C6 roots merge to form the upper trunk, C7 root forms the middle trunk, and C8 and T1 roots merge to form the lower trunk.
Narakas classified NBPP into 4 grades (8):
Grade I - have injury to C5 and C6 (upper trunk), resulting in weakness of shoulder abduction, external rotation, and elbow flexion.
Grade II - also have injury to C7 (middle trunk with additional weakness of the elbow, wrist, and finger extension.
Grade III - have a panplexopathy with injury to C5, C6, C7, C8 and T1 and a flail arm.
Grade IV - patients have injuries similar to Grade II, but also have Horner syndrome.
Patterns and Early Interventions
Narakas Grade I and II patients have been shown to have a higher likelihood of spontaneous recovery (6). In this study (6), authors also identified the peripartum and neonatal factors that were associated with persistent NBPP. The conclusion was cephalic presentation, induction or augmentation of labor, birth weight >9 lbs, and the presence of Horner syndrome all significantly increased the odds of persistence at 1 year, which cesarean delivery and Narakas Grade I to II injury significantly reduced the odds of persistence.
Persistent NBPP is debilitating, affecting function, development, and quality of life condition. In these patients, early referral to, and early evaluation by interdisciplinary specialty clinics, is paramount to maximizing recovery. Of significant importance for maximizing outcomes is determining which patients are likely to recover spontaneously and which patients are likely to benefit from timely surgical nerve reconstruction in the context of multidisciplinary treatment paradigms. One paradigm that has been used is the assessment of biceps function at 3 months, with the lack of recovery signifying a poor prognosis and serving as an indication for surgery (9). Other paradigms have focused on earlier evaluation of biceps and triceps function coupled with electrodiagnostic or other functional testing such as the "towel test" or "cookie test" (10).
A number of paradigms have been developed in an attempt to create a decision-making protocol, but none has become the standard of care. Most agree that when a neurotraumatic lesion or nerve root avulsion can be identified, that is an indication for early nerve surgery (9). However, in the absence of a neurotmetic lesion or root avulsion, practice varies. Most management strategies have focused on evaluation by physical examination, or functional examination using the progress in the examination over time to dictate whether operative management is pursued. Impaired hand function at 2 to 3 months of age is seen by some as an indication for surgery (11). Operative management is also often recommended for those infants who lack shoulder external rotation and elbow flexion by 3 to 4 months of age (12).
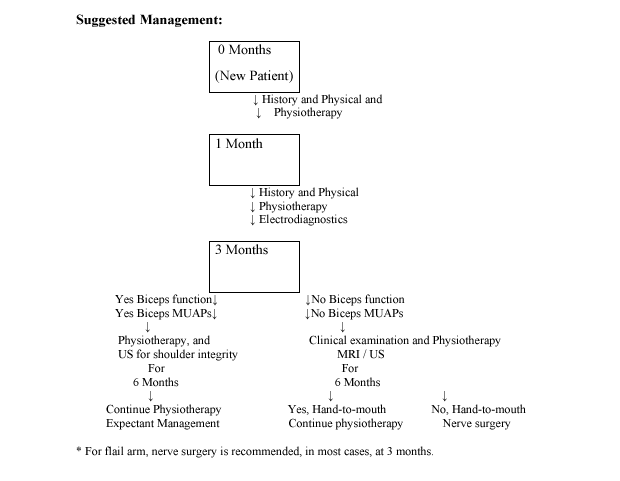
Abbreviations: MUPPs (motor unit action potentials); US (Ultrasound); MRI (magnetic resonance imaging)
Electrodiagnostic medicine, is a specific area of physical medicine and rehabilitation in which a physiatrist uses clinical history, physical examination, and the techniques of electrophysiologic study to diagnose and treat neuromuscular disorders. The roles of electrodiagnosis include:
- Determining the localization and distribution of a lesion and severity of a disease;
- Characterizing the evolution of a disease;
- Estimating prognosis;
- Differentiating neuropathy and myopathy; and
- Monitoring the response to a treatment.
For these to be achieved, a logical approach during every step of testing is needed, followed by data interpretation and clinical correlations.
Temporal Trends
Epidemiological knowledge of the incidence, prevalence, and temporal changes of NBPP should assist the clinician, avert unnecessary interventions, and help formulate evidence-based health policies. NBPPs are notable for six things (13).
- Frist, the rate of NBPP in the US and other countries is comparable: 1.5 vs. 1.3 per 1,000 total births, respectively.
- Second, the rate of NBPP may be decreasing: 0.9, 1.0 and 0.5 per 1,000 births for publications before 1990, 1990 - 2000, respectively.
- Third, the likelihood of not having concomitant shoulder dystocia with NBPP was 76% overall, though it varied by whether the publication was from the US (78%) vs. other countries (47%).
- Fourth, the likelihood of NBPP being permanent (lasting at least 12 months) was 10 - 18% in the US based reports and 19 - 23% in other countries.
- Fifth, in studies from the US, the rate of permanent NBPP is 1.1 - 2.2 per 10,000 births and 2.9 - 3.7 per 10,000 births in other nations.
- Sixth, the authors estimated that approximately 5,000 NBPPs occur every year in the US, of which over 580 - 1,050 are permanent, and that since birth, 63,000 adults have been afflicted with persistent paresis of their brachial plexus.
The exceedingly infrequent nature of permanent NBPP necessitates a multi-center study to improve our understanding of the antecedent factors and to abate the long-term sequalae.
Multidisciplinary Conservative Treatment
Conservative treatment of NBPP involves early diagnosis and follow-up, if possible, within two to three weeks after child's birth (14). Conservative treatment should involve a multidisciplinary team, composed of physiatrists, clinical neurophysiologist, neurosurgeons, occupational therapists, and physiotherapists. The treatment administered by the physiotherapist and occupational therapist involves smooth joint movements and sensory stimulation, such as passive/active mobilization exercises, stretches, tactile stimulation with different textures, vibration, and brushing techniques to promote sensory ability in the injured limb, and bimanual activities. Sensory stimulation is as important as motor stimulation and can consist in suckling any finger on the injured limb and stimulating the skin with different textures, temperatures, and vibrations (15). Electrical stimulation/electrostimulation is a complementary means to technique used in conservative therapies for the rehabilitation of brachial plexus palsy, that promotes gaining muscle tone/strength on the affected muscles, and significant improvements in the mobility of the injured limb. These therapies aim to ensure the conditions needed for the functional recovery of the limb following nerve regeneration, which implies the prevent of muscle shrinkage, sagging, joint deformities, and muscle contractures.
Both therapies play a key role in the rehabilitation of neonatal brachial plexus palsy, but it is essential to involve parents in the rehabilitation program, so that professionals and family members work jointly. Therapy should be administered several times a week and, at home as frequently as possible, for example at each meal or with every nappy change (15). Most studies reveal that conservation treatment performed by therapists significantly reduces injuries removing the need for surgical intervention.
Conservative/Surgical Treatment
There are different tools that are used as means and/or complementary techniques to the conservative/surgical treatment of NBPP, such as electrostimulation, botulinum toxin injection, thermoplastic splints, posterior and anterior temporary splints (for physiological positioning, facilitating functional motor function, and preventing vicious posture. Anterior and posterior fist or hand splints control and prevent, at the fist level, extreme ulnar flexion, and deviation. Anterior splints can simultaneously control thumb adduction, and posterior splints allow more freedom of child's palm), and constraint induced movement therapy.
Electrostimulation is commonly used to increase muscle strength, with the aim of promoting functional muscle recovery after nerve injury. It inhibits muscle atrophy during the reinnervation period, and accelerates nerve regeneration, resulting in improved muscle strength and range of motion in the injured limb. The benefits of electrostimulation in the recovery of NBPP as a complement to conservative/surgical treatment are evident.
The use of temporary immobilizing splints is indicated for children with impaired wrist function, which can help improve hand function, and prevent wrist drop, thus promoting wrist extension. Some splints are used during sleep, and other more functional ones are used during awake time activities (16).
Injection of botulinum toxin into healthy antagonist muscles have proven effective in the treatment of muscle imbalances, co-contractions, and muscle contractures in children with NBPP. The aim of this complementary treatment option is to balance strength and to allow the affected muscles to develop by adapting the movement pattern to the ongoing nerve recovery (17). Studies on the benefits of using botulinum toxin for treating NBPP demonstrate benefits for the elbow function, with improved flexion and supination (17).
Constraint induced movement therapy demonstrates that performing activities at home for one hour a day can improve mobility, functional capacity, speed, range of motion, and hand manipulation ability. Other studies reveal the effectiveness of movement therapy, leading to improvements in mobility, increased predisposition to use the injured limb, and frequency of use.
Surgical Treatment
Surgical nerve reconstruction may be necessary for rehabilitating patients with NBPP, especially children who do not show spontaneous recovery during the first months of life. However, conservative treatment should be favored whenever possible. When surgical intervention is required, both primary and secondary microsurgeries are available. Primary microsurgery techniques include recession and reconstruction of the neuroma, neurolysis, and nerve transfer. Studies reveal that, as a primary surgery of NBPP, neurolysis combined with nerve transfer produces good results (18).
Nerve transfer surgeries reconnect nerves that have less important roles or are redundant with the target nerve, without intervention (16). In situations where the lesion affects the suprascapular nerve, shoulder function is impaired (abduction and external rotation). Grafts extracted from the proximal C5 root stump or accessory nerve are often used to reconstruct the suprascapular nerve. The use of the phrenic nerve has also been shown to provide a similar level of recovery to the use of the median nerve, increasing the number of graft options available to recover suprascapular nerve function (19).
When the C5 and C6 nerve roots are affected, i.e., in Erb's palsy, affecting shoulder abduction and external rotation, elbow flexion, and forearm supination, and when there is no evidence of spontaneous recovery, surgery is a valid treatment option. The Oberlin's procedure involves the transfer of the ulnar nerve to the cutaneous nerve and is an effective way of recovering the elbow function, improving elbow flexion, and leading to increase functional use of the affected limb (20). Other studies corroborate the positive result in the recovery of the biceps function with the Oberlin's procedure (20).
Another alternative for treating Erb's palsy is to extract a graft from the median nerve and use it to reconstruct the biceps nerve, which has been shown to improve elbow function (16). The use of the ulnar or median nerves as grafts for treating the biceps are viable options, not only because the evidence supporting the recovery of the biceps function, but also due to the proximity of the biceps nerves.
Secondary surgery involves tendon transfer, arthrodesis, or osteotomies, and may be an option for children who have partially recovered after primary surgery but still show some deficits deemed treatable, or for children who experience spontaneous recovery but still show some functional deficits. In summary, primary surgery is the initial treatment option for children who do not experience spontaneous recovery, and secondary surgery aims to promote the functional improvement of the limbs. Other authors report that secondary surgery may include procedures to improve external shoulder rotation, such as the Hoffer's procedure, and the Steindler flexorplasty to improve elbow flexion. Secondary surgery is also used to improve hand function, but relevant studies suggest that improving hand function is a great challenge, and the results of these surgeries are merely palliative in most cases.
Criteria for Conservative and/or Surgical Treatments
Criteria for opting between conservative treatment or surgical treatment for neonatal brachial plexus are not consensual. There is no scientific evidence favoring surgical treatment/nerve reconstruction over conservative treatment. Children who do not show spontaneous recovery during the first months of life are considered suitable recipients of reconstructive nerve surgery.
Some studies defend that primary surgery (neuroma excision or nerve graft) is indicated for children who do not have biceps function (elbow flexion against gravity) at the age of 3 months. If at this age there is evidence of nerve root avulsion, surgery is indicated. Some authors defend that the decision to operate can be postponed until 5 to 6 months if there is no biceps function at the age of 3 months, but some level of shoulder recovery is observed (21).
Some studies defend that if there is some level of recovery of the biceps function at the age of 3 months, the situation can be reassessed between the age of 6 and 9 months to assess whether there is need for surgical intervention. Other authors corroborate the influence of biceps recovery in opting for conservation/surgical treatment, stating that biceps recovery before the age of 3 months is a predictor of complete or near-complete shoulder recovery. The exact moment to decide on nerve reconstruction is difficult to identify, but a possible range is established between the child's third and sixth month of life (22).
Prognosis
Neonatal brachial plexus palsy is complex, present with many different severity levels and prognoses, as well as reinnervation and recovery patterns, which are unpredictable factors that make it harder to define rigorous criteria for reconstructive surgery. Several scientific studies reveal how difficult it is to opt for surgical treatment of NBPP. For severe lesions, with avulsion and rupture of the nerve roots, the neurological prognosis without surgical intervention is poor, justifying surgical treatment. Another strong indicator for surgery is decreased hand function without spontaneous recovery. For children that resent with partial lesions at the C5 - C6 or C5 - C6 - C7 level, surgery is a "grey area", as different levels of spontaneous recovery can take place, leading to the decision for surgery at different stages during the child's life, namely at 3 months of age, 5 to 6 months, or up to 9 months.
An early rehabilitation treatment based on intensive multidisciplinary conservative treatment can lead to favorable functional outcomes in children whose biceps recover spontaneously between the ages of 3 and 6 months. When there is no spontaneous recovery or complete paralysis of the limb, the most widely prescribed treatment is the surgical treatment.
Infants with more severe NBPP benefit from primary nerve surgery to improve function. The timing of the surgery, however, is controversial. The Treatment and Outcomes of Brachial Plexus Injury (TOBI) study is a multicenter prospective study with the primary aim of determining the optimal timing of this surgical intervention (23). This study compared outcomes evaluated 18 to 36 months after "early" microsurgery (at <6 months of age) with the outcomes of "late" microsurgery (at >6 months of age). The conclusion was that surgery earlier in infancy (at a mean age of 4.2 months) does not lead to better postoperative outcomes of NBPP nerve surgery than when the surgery is performed later in infancy (mean age of 10.7 months).
NBPP and Medicolegal Considerations
Perinatal disorders are prone to malpractice litigation. NBPP results from stretching the nerves in the perinatal period and may lead to paresis or paralysis and sensory loss in the affected arm. Unless NBPP is resolved, children are followed for at least two years. Though there were no differences in peripartum events, in this study, almost 1 out of 2 children managed by interdisciplinary team had concomitant litigation (24). The only factor associated with litigation was having brachial nerve surgery. Efforts are warranted to avert NBPP and mitigate litigations. In this study (24), litigation was pursued by 48%. Comparing families that litigated versus those that did not, there were no differences in demographics, peripartum characteristics, operative versus spontaneous vaginal birth, shoulder dystocia in current pregnancy or birth weight above 9 lbs. The number of children having brachial nerve surgery was significantly higher among families that litigated (46%) versus those that did not.
Psychosocial factors are an important aspect of parent and patient-reported outcomes in NBPP. Litigation is associated with worse parent reports of childrens function and pain following NBPP, independent of age, injury severity, and the patients' own report of their function. Litigation status should be considered a cofounding variable in the use of parent-reported outcomes in neonatal brachial plexus injury research. Parents involved in litigation may benefit from additional support.
Lack of physician-patient communication can be a key factor associated with malpractice litigation in NBPP. Physician-controllable factor, such as communication in the perinatal period, are associated with malpractice litigation in NBPP (25). The perceived level of global disability may affect the pursuit of malpractice litigation, whereas the isolated extent of nerve root involvement and/or upper extremity dysfunction are not significant factors in pursuing litigation. Identifying and ameliorating these factors within the practice environment may decrease the animosity between families and health care providers and improve overall outcome for patients with NBPP.
Managing Macrosomia
Suspected macrosomia is encountered commonly in obstetric practice. A variety of maternal factors predispose a newborn to macrosomia, including constitutional factors, preexisting diabetes, and gestational diabetes mellitus (GDM), maternal pre-pregnancy obesity, excessive gestational weight gain, abnormal fasting and post-prandial glucose levels, dyslipidemia, a prior macrosomic newborn (weight more than 4,000 g), and post-term pregnancy (26). The interplay of these risk factors is complex and varies by pre-pregnancy body mass index (BMI), race and ethnicity.
Diagnosis: An accurate diagnosis of macrosomia can only be made by weighing the newborn after birth. The prenatal prediction of newborn birth weight is imprecise. Ultrasonography enables the direct measurement of various fetal body parts, but its accuracy in predicting macrosomia is poor. A meta-analysis of 29 studies found a sensitivity of 56% and specificity of 92% for predicting birth weight more than 4,000 g (27). Ultrasound accuracy decreases with increasing fetal weight beyond 4,000 g such that an ultrasound-estimated fetal weight more than 4,500 g accurately predicts birth weight more than 4,500 g in only 33 - 44% of cases (27).
Small studies of 3-D ultrasonography have shown mixed results. A formula using biacromial diameter and a macrosomic specific formula have shown high rates of accuracy but are not yet reliable (28). Magnetic resonance imaging (MRI) has been shown to have higher sensitivity and specificity than ultrasonography, but given its cost and discomfort, as well as its size limitations for obese women. Further study is needed to determine the appropriate clinical use of MRI in this setting (29).
Interventions for Treating or Preventing Suspected Macrosomia: Interventions shown to reduce macrosomia include exercise during pregnancy, low glycemic diet in women with GDM, and pre-pregnancy bariatric surgery in women with class 2 or class 3 obesity. Given the health benefits, particularly for pregnancy outcomes, pre-pregnancy counseling of morbidly obese patients regarding the benefits and risks of bariatric surgery is recommended. Women without contraindications should be encouraged to engage in aerobic and strength-conditioning exercises during pregnancy to reduce the risk of macrosomia.
Control of maternal hyperglycemia reduces the risk of macrosomia; therefore maternal glucose management is recommended for pregnancies complicated with diabetes. Diets that include additional dietary fiber further decrease the risk.
Mode of Delivery - Vaginal delivery vs. Cesarean Section
Cesarean birth reduces, but does not eliminate, the risk of birth trauma and NBPP associated with macrosomia (30). Although the prediction of macrosomia in imprecise, scheduled cesarean birth may be beneficial for newborns with suspected macrosomia who have an estimated fetal weight of at least 5,000 g in women without diabetes and an estimated fetal weight of at least 4,500 g in women with diabetes. However, given the absence of randomized clinical trials, planned cesarean birth for suspected macrosomia is controversial and is based on expert opinion.
Most fetuses with macrosomia who are delivered vaginally do not experience shoulder dystocia. Consequently, if all fetuses suspected of being macrosomic had a cesarean birth, the cesarean birth rate would increase disproportionately to the reduction in the rate shoulder dystocia. Prolonged first and second stages of labor are common when macrosomia is present, leading to increased rate of conversion to cesarean during labor. Whether to conduct an operative vaginal delivery in cases of suspected macrosomia is another important consideration. Whether forceps delivery of a macrosomic newborn increases the risk of shoulder dystocia or not, is unclear. The risk of shoulder dystocia at the time of operative vaginal delivery increases when more risk factors are present. For example, when an LGA newborn, diabetes, and vacuum delivery were all present, the odd ratio (OR) was 33 (31). Thus, the clinician should have a heightened awareness for shoulder dystocia in these situations, although judicious use of operative vaginal delivery is reasonable even when risk factors are present.
The patient should be counseled regarding these risk, caution should be exercised, and preparations should be made for the possibility of encountering shoulder dystocia. Suspected fetal macrosomia or LGA fetus is not an indication of labor before 39 0/7 weeks of gestation because there is insufficient evidence that benefits of reducing shoulder dystocia risk would outweigh the harms of early delivery (32). Pregnant women with macrosomia should be provided individualized counseling about the risk and benefits of vaginal births and cesarean births based on the degree of suspected macrosomia, accounting for their relevant clinical considerations. It is appropriate for patients, obstetricians and gynecologists, and other obstetric care providers to consider past and predicted birth weights when making decisions regarding labor after cesarean however, suspected macrosomia is not a contraindication to labor after cesarean.
Multidisciplinary Shoulder Dystocia Drills
Regularly perform multidisciplinary shoulder dystocia drills at your institution. The Joint Commission recommends that clinical drills be performed to help staff prepare for high-risk labor and delivery events, including shoulder dystocia, emergency cesarean delivery, and maternal hemorrhage (33). When shoulder dystocia occurs, extensively chart the event and interventions used. World Health Organization (WHO) has developed a patient safety check-list focused on key clinical elements in the antepartum, intrapartum, and postpartum periods and overall timing of the delivery to document when a shoulder dystocia occurs.
Our recommendation is stop using the term "traction" in the medical records and in obstetric literature. Words are meaningful and open to multiple interpretations. Often, words have unintended consequences. Plaintiff attorneys often highlight the obstetrician's use of "traction" or "excessive traction" as the cause of NBPP. Orthopedic surgeons and pediatricians often state in their records that the NBPP was a "traction injury," further supporting the plaintiff attorney's contention that excessive traction applied by the healthcare provider delivering the newborn caused the traction injury. Our suggestion and usage is gentle downward guidance to deliver the fetal shoulders and body.
In a high-risk situation, proceed quickly to delivery of the posterior arm. When you recognize a shoulder dystocia in a high-risk situation (maternal diabetes and large fetus), it may be wise to move quickly to delivery of the posterior arm. In high-risk situations, delivery of the posterior arm is the maneuver with the greatest likelihood of resolving a severe shoulder dystocia, with the least force applied to the brachial plexus that is trapped under the mother's symphysis pubis.
Practice, and then practice some more. A difficult-to-resolve shoulder dystocia is one of the most dramatic and frightening obstetric events. We know that we will all experience such cases. If we prepare well and frequently practice shoulder dystocia maneuvers, the dread of being responsible for resolving a shoulder dystocia, will diminish. In most cases we will be able to report "mother and newborn safely birthed."
Summary
The knowledge about NBPP is continually evolving. What is known at this time with reasonable certainty is that NBPP occurs infrequently and can be caused by maternal (endogenous) forces or clinician-applied (exogenous) forces or a combination of both. The primary objective in the presence of clinically recognizable shoulder dystocia continues to be the delivery of fetus before fetal experiences hypoxic-ischemic injury. Any intervention to affect delivery must necessarily balance the risk of using ancillary maneuvers, which will increase strain on the fetus' brachial plexus, against the risk of hypoxic-ischemic brain injury. The birth attendant is the individual best equipped to assess this balance and decide on the type and degree of intervention. NBPP can occur with or without associated, clinically recognizable shoulder dystocia. Finally, in the presence of shoulder dystocia, all intervention by way of ancillary maneuvers - no matter how expertly performed - will necessarily increase strain on the brachial plexus.
In general, with regard to surgical treatment, primary surgery includes surgical procedures involving nerve transfer, and the ulnar, median, and phrenic nerves are used as grafts/donors in this type of surgery. Secondary surgeries are used in patient, show after primary surgery, have reached a certain level of recovery, but still show some deficits considered treatable, or in patients who have not undergone primary surgery and show some type of spontaneous recovery but still have deficit.
Significant advances have been made in the science of biomedical engineering that have informed this document and that will continue to increase the understanding of the mechanical forces that affect the fetus during labor and delivery. In the years to come, the greater sophistication of physical and computer models will enhance the ability to assess the effects of both endogenous forces of labor and delivery and exogenous forces generated by the birth attendant in facilitating an obstructed delivery.
Suggested Reading
Shoulder Dystocia
http://www.womenshealthsection.com/content/obs/obs007.php3
References
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep 2018;67(8):1-50. (Level II-3)
- Doty MS, Chen HY, Sibai BM, Chauhan SP. Maternal and neonatal morbidity associated with early term delivery of large-for-gestational-age but nonmacrosomic neonates. Obstet Gynecol 2019;133:1160-1166. (Level II-2)
- American College of Obstetricians and Gynecologists (ACOG). Report of the ACOG Task Force on Neonatal Brachial Plexus Palsy; Executive Summary. Obstet Gynecol 2014;123:902-904
- Hammad IA, Chuhan SP, Ghermen RB, et al. Neonatal brachial plexus palsy with vaginal birth after cesarean delivery: a case-control study. Am J Obstet Gynecol 2013;208:229.e1-5. (Level II-2)
- Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol 2000;182(3):689-691
- Wilson TJ, Chang KWC, Chauhan SP, Yang LJS. Peripartum and neonatal factors associated with the persistence of neonatal brachial plexus palsy at 1 year: a review of 382 cases. J Neurosurg Pediatr 2016;17:618-624
- Pondaag W, Malessy MJA, van Dijk JG, Tommeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol 2004;46(2):138-144
- Yang LJ. Neonatal brachial plexus palsy - management and prognostic factors. Semin Perinatol 2014;38:222-234
- Malessy MJ, Pondaag W. Nerve surgery for neonatal brachial plexus palsy. J Pediatr Rehabil Med 2011;4:141-148
- Okby R, Sheiner E. Risk factors for neonatal brachial plexus paralysis. Arch Gynecol Obstet 2012;286:333-336
- Pondaag W, Malessy MJ. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J Neurosurg 2006;105:1Suppl33-40
- Pondaag W, de Boer R, van Wijlen-Hempel, et al. External rotation as a result of suprascapular nerve neurotization in obstetric brachial plexus lesions. Neurosurgery 2005;57:530-537
- Chauhan SP, Blackwell SB, Ananth CV. Neonatal brachial plexus palsy: incidence, prevalence, and temporal trends. Semin Perinatol 2014;38(4):210-218
- Yanes SVL, Sandobal FEC, Camero AD, Ojeda DL. Obstetric brachial plexus palsy in the context of early physical rehabilitation. Medi Sur2014;12:635-649
- Smith B, DAunter A, Yang L, Wilson T. An update on the management of neonatal brachial plexus palsy replacing old paradigms: a review. JAMA Pediatr 2018;172:585-591
- Frade F, Gomez-Salgado J, Florindo-Silva F. Rehabilitation of neonatal brachial plexus palsy: Integrative literature review. J Clin Med 2019;8(7):980-993
- Shin YB, Shin MJ, Chang JH, et al. Effects of botulinum toxin on reducing the co-contraction of antagonists in birth brachial plexus palsy. Ann Rehabil Med 2014;38:127-131
- Lin JC, Schwentker-Cozza A, Curtis CG, Clarke HM. Final results of grafting versus neurolysis in obstetrical brachial plexus palsy. Plast Reconstr Surg 2009;123:939-948
- Al-Qattan MM, El-Sayed AAF. The use of the phrenic nerve communicating branch to fifth cervical root for nerve transfer to the suprascapular nerve in infants with obstetric brachial nerve plexus palsy. Biomed Res Int 2014;1-4. Doi: 10.1155/2014/153182
- Figueiredo RM, Grechi G, Gepp RA. Oberlin's procedure in children with obstetric brachial plexus palsy. Childs Nerv Syst 2016;32:1085-1091
- Bade S, Lin J, Curtis C, Clarke H. Extending the indications for primary nerve surgery in obstetrical brachial plexus palsy. Biomed Res Int 2014;1-5. doi: 10.1155/2014/627067
- Chin KF, Misra VP, Sicuri GM, et al. Intra-operative neurophysiological prediction of upper trunk recovery in obstetric brachial plexus palsy with neuroma incontinuity. Bone Joint J 2013;95:699-705
- Bauer AS, Kalish LA, Adamczyk MJ, et al. Microsurgery for brachial plexus injury before versus after 6 months of age: Results of the multicenter Treatment and Outcomes of Brachial Plexus Injury (TOBI) Study. J Bone Joint Surg Am 2019;Nov. 26. doi: 10.2106/JBJS. 18.01312. [Epub ahead of print] PMID: 31770293
- Chauhan SP, Chang KWC, Ankumah NE, Yang LJS. Neonatal brachial plexus palsy: obstetric factors associated with litigation. J Matern Fetal Neonatal Med 2017;30(20):2428-2432
- Domino J, McGovern C, Chang KW, Carlozzi NE, Yang LJ. Lack of physician-patient communication as a key factor associated with malpractice litigation in neonatal brachial plexus palsy. J Neurosurg Pediatr 2014;13(2):238-242
- Beta J, Khan N, Khalil A, et al. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;54:308-318
- Milner J, Arezina J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: a systematic review. Ultrasound 2018;26:32-41
- Youssef AEA, Amin AF, Khalaf M, Khalaf MS, et al. Fetal biacromial diameter as a new ultrasound measure for prediction of macrosomia in term pregnancy: a prospective observational study. J Matern Fetal Neonatal Med 2019;32:2674-2679. (Level II-2)
- Kadji C, Cannie MM, Resta S, Guez D, et al. Magnetic resonance imaging for prenatal estimation of birthweight in pregnancy: review of available data, techniques, and future perspectives. Am J Obstet Gynecol 2019;220:428-439
- American College of Obstetricians and Gynecologists (ACOG). ACOG Committee Opinion No. 765. Avoidance of nonmedically indicated early-term deliveries and associated neonatal morbidities. Obstet Gynecol 2019;133:e156-163. (Level III)
- 3Dall'Asta A, Ghi T, Pedrazzi G, Frusca T. Does vacuum delivery carry a higher risk of shoulder dystocia? Review and meta-analysis of the literature. Eur J Obstet Gynecol Reprod Biol 2016;204:62-68
- American College of Obstetricians and Gynecologists (ACOG). ACOG Practice Bulletin. Macrosomia. Number 216. Obstet Gynecol 2020;135:e18-e34
- The Joint Commission. Sentinel Event Alert, Issue 30: Preventing Infant Death and injury during delivery. https://www.jointcommission.org/resources/patient-safety-topics/sentinel-event/sentinel-event-alert-newsletters/sentinel-event-alert-issue-30-preventing-infant-death-and-injury-during-delivery/ Last accessed 1 March 2020
Published: 6 June 2020
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


