Vitamin K Deficiency Bleeding
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Vitamins are substances our bodies need, which we get from either the food we eat or from a multivitamins. Vitamins are normally stored in the body. A person without enough of a vitamin stored in the body is "vitamin deficient" or has a "vitamin deficiency." Vitamin K is a substance that our body needs to form clots and to stop bleeding. We get vitamin K from the food we eat. Some vitamin K is also made by the good bacteria that live in our intestines. Babies are born with very small amounts of vitamin K stored in their bodies, which can lead to serious bleeding problems if not supplemented.
The purpose of this document is to review new aspects of vitamin K-deficiency bleeding (VKDB) and prophylaxis. VKDB is rare, unpredictable, and life-threatening condition. Warning signs such as minimal bleeds, evidence of cholestasis, and failure to thrive often are present but overlooked. The risk of VKDB are minimized if prophylaxis recommendations are followed and if warning signs are recognized and promptly acted upon. The next goal is the search for methods of identifying early. Few infants are destined to bleed, so that targeted prophylaxis can replace the current "prophylaxis for all."
Definition
Vitamin K Deficiency bleeding (VKDB) is defined as bruising, bleeding, or intracranial hemorrhage in infants younger than 6 months, not due to other coagulopathies, in combination with normalization of the coagulopathy (partial thromboplastin time [PT] or activated partial thromboplastin time[APTT]) after administration of vitamin K (1).
Introduction
Vitamin K deficiency can cause severe bleeding in breastfed infants owing to insufficient amounts of vitamin K in breast milk. The absorption of vitamin K is strongly dependent on the intestinal bile acids. Diminished or absent intestinal delivery of bile, which occurs during cholestasis, puts infants especially at risk for malabsorption of vitamin K, and other fat-soluble vitamins. Infants who have been breastfed exclusively are at the highest risk for late VKDB, particularly if the cholestasis has not yet been diagnosed. Many countries have introduced prophylactic regimens of vitamin K supplementation to prevent VKDB. The optimal dose, route, and frequency of administration of vitamin K, however, are still unclear.
"Vitamin K," the generic name for a family of compounds with a common chemical structure of 2-methyl-1, 4-naphthoquinone, is a fat-soluble vitamin that is naturally present in some foods and is available as a dietary supplement (2). These compounds include phylloquinone (vitamin K1) and a series of menaquinones (vitamin K2). Menaquinones have unsaturated isoprenyl side chains and are designated as MK-4 through MK-13, based on the length of their side chain (3). MK-4, MK-7, and MK-9 are the most well-studied menaquinones.
Vitamin K functions as a co-enzyme for vitamin K-dependent carboxylase, an enzyme required for the synthesis of proteins involved in hemostasis (blood clotting) and bone metabolism, and other diverse physiological function (4). Prothrombin (clotting factor II) is vitamin K-dependent protein in plasma, that is directly involved in blood clotting. Warfarin (Coumadin®) and some anticoagulants used primarily in Europe antagonize the activity of vitamin K, and in turn, prothrombin (5). For this reason, individuals who are taking these anticoagulants need to maintain consistent vitamin K intakes.
Risk Factors
All infants, regardless of sex, race or ethnic background, are at higher risk of VKDB until they start eating regular foods, usually at age 4-6 months, and until the normal intestinal bacteria start making vitamin K. This is because (6):
- At birth, babies have very little vitamin K stored in their bodies because only small amounts pass to them through the placenta from their mothers.
- The good bacteria that produce vitamin K are not yet present in the newborn's intestines. Poor intestinal absorption of vitamin K and immature gut flora.
- Breast milk contains low amounts of vitamin K, so exclusively breast-fed babies don't get enough vitamin K from the breast milk, alone.
- Low activity level of vitamin K epoxide reductase.
Some things can put infants at a higher risk for developing VKDB. Babies at greater risk include:
- Babies who do not receive a vitamin K shot at birth. The risk is even higher if they are exclusively breastfed.
- Babies whose mothers used certain medications, like isoniazid or medicines to treat seizure. These drugs interfere with how the body uses vitamin K.
- Babies who have liver disease; often they cannot use the vitamin K their body stores.
- Babies who have diarrhea, celiac disease, or cystic fibrosis often have trouble absorbing vitamins, including vitamin K, from the foods they eat.
Types and Prevalence
Since babies can be affected until they are 6 months old, healthcare providers divide VKDB into three types; early, classical and late.
- Early and classical VKDB are more common, occurring in 1 in 60 to 1 in 250 newborns, although risk is much higher for early VKDB among those infants whose mothers used certain medications during the pregnancy (7).
- Late VKDB is rarer, occurring in 1 in 14,000 to 1 in 25,000 infants (8).
- Infants who do not receive a vitamin K shot at birth are 81 times more likely to develop late VKDB than infants who do receive a vitamin K shot at birth (9).
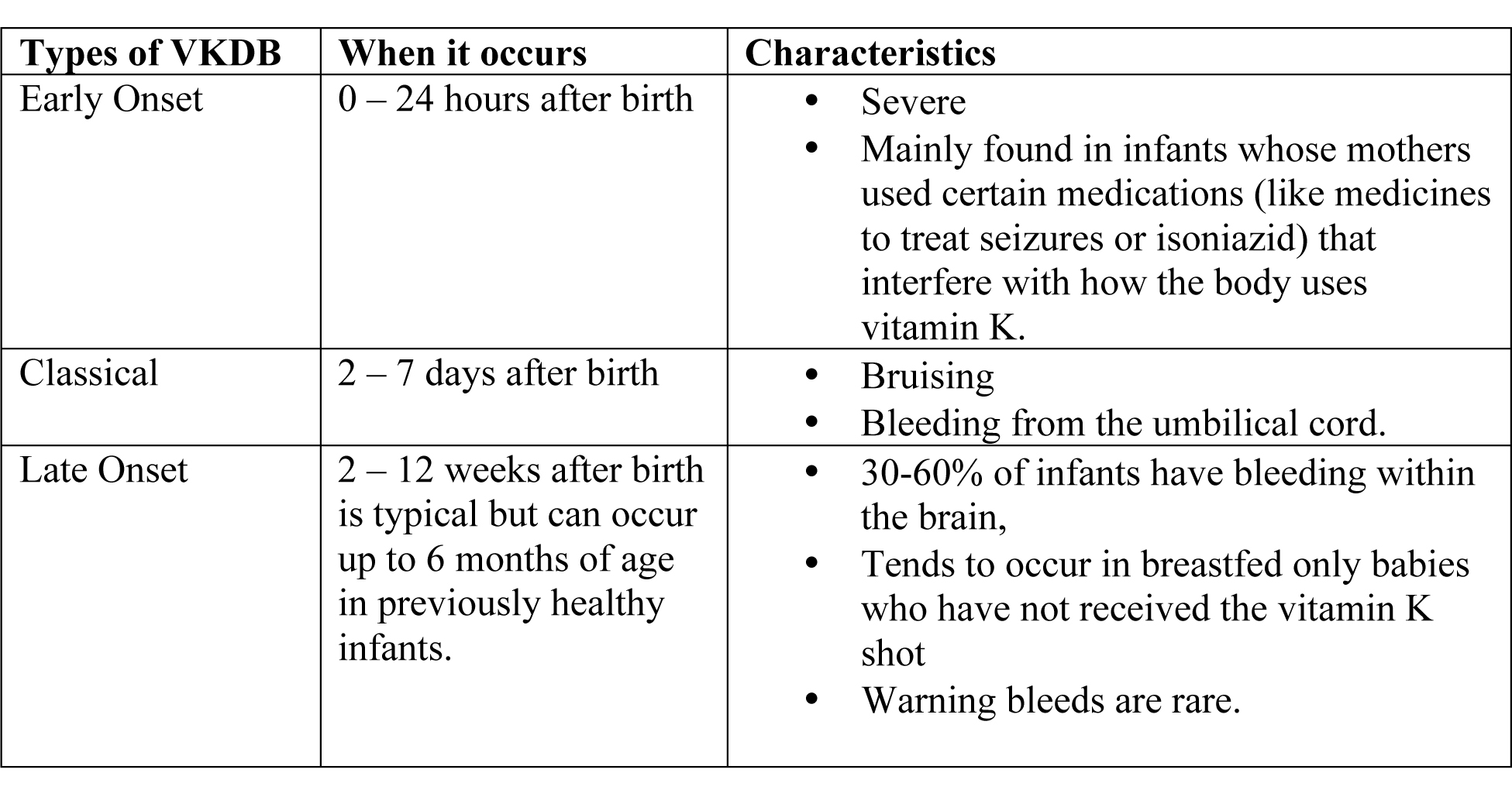
Table 1. Types of vitamin K deficiency bleeding in newborns.
Early Onset VKDB
It is commonly associated with maternal malabsorption disorders and medications that inhibit the activity of vitamin K, such as antiepileptics (carbamazepine, phenytoin, and barbiturates), anti-tuberculosis medications (isoniazid and rifampicin), certain antibiotics (cephalosporins), and vitamin K antagonists (warfarin); moreover, it is more common in pregnant women who do not receive vitamin K prophylaxis before delivery (10). Recent studies have revealed that placental functions would be impaired in cases of pre-eclampsia and intrauterine growth restriction. These conditions affect the placental transfer of vitamin K (11). The incidence of early onset VKDB in at-risk neonates who did not receive vitamin K supplementation reportedly varied from 6% to 12% (12). Among cases that did not receive vitamin K administration before delivery, the occurrence of early onset VKDB is frequent, and the prognosis of such infants is poor because VKDB has a high incidence of intracranial hemorrhage (13).
Classic VKDB
It is associated with low vitamin K content in breast milk, poor feeding of milk, and/or inadequate vitamin K prophylaxis. It has good prognosis because its bleeding sites are typically located in the intestinal tract. In addition, a natural decrease in the activity of prothrombin during this period has been reported. Without vitamin K prophylaxis, classic VKDB is reported to occur in 0.25% to 1.7% of neonates without underlying diseases (14).
Late Onset VKDB
It occurs between 2 weeks and 6 months after birth, with an increased occurrence reported between 3 and 8 weeks after birth (15). It has an incidence of 4.4 to 72.0 per 100,000 live births in Asia and Europe. The late-onset VKDB is classified as idiopathic without risk factors of vitamin K deficiency except for breastfeeding or as secondary VKDB with risk factors of vitamin K deficiency, such as malabsorption or cholestasis, because vitamin K absorption closely depends on the intestinal availability of bile (16).
The hemorrhagic manifestations of late-onset VKDB mainly involve the gastrointestinal tract, skin, and central nervous system and present as intracranial hemorrhage. Most reported cases of late-onset have presented with intracranial hemorrhage. Late-onset VKDB is more frequent in the Asian population than the Caucasian population (17). This finding may be partially explained by dietary habits or the 6-fold higher incidence of biliary atresia in Asia than in Western Europe (18). Recent studies have confirmed that genetic variants of 10q24 are closely associated with the epidemiology of biliary atresia.
Late-onset VKDB affects morbidity and mortality, with the mortality being as high as 20% to 50% (19). In a Japanese survey including 427 patients with idiopathic VKDB, 62 patients - 14.5% died and 171 - 40% survived with severe neurological sequelae (19).
Diagnosis of VKDB in Infancy
Unfortunately, in the majority of cases of VKDB, there are no warning signs before a life-threatening event starts. Babies with VKDB might develop any of the following signs:
- Bruises, especially around the baby's head and face;
- Bleeding from the nose or umbilical cord;
- Skin color that is paler than before. For darker skinned babies, the gums may appear pale;
- After the first 3 weeks of life, the white parts of your baby's eyes may turn yellow.
- Stool that has blood in it, is black or dark and sticky (also called 'tarry'), or vomiting blood;
- Irritability, seizures, excessive sleepiness, or a lot of vomiting may all be signs of bleeding in the brain.
The diagnosis of VKDB is commonly indicated by a prolonged activated partial thromboplastin time (APTT) and prothrombin time (PT). VKDB is characterized by a PT international normalized ration (INR) >4 or a value >4 times the normal values in the presence of normal platelet count and fibrinogen level. The diagnosis is confirmed based on the increased levels of proteins induced by vitamin K absence or antagonists (PIVKAs) and a rapid normalization of coagulation parameters, such as APTT and PT, after vitamin K administration or both.
Although the serum vitamin K level is a useful status indicator, its use is not practical because of the technical difficulty involved (20). Reference values in healthy adults range from 0.2 to 1.0 μg/L (median, ~0.5 μg/L). The screening test program was substituted for prophylactic vitamin K administration because heel punctures are more invasive, difficult, and expensive than vitamin K administration (21).
Several factors have revealed that genetic factors can influence vitamin K-dependent coagulation system and intravascular hemorrhage (22). Further studies regarding the usefulness of testing for genetic variants to prevent and diagnose VKDB are warranted.
Vitamin K Prophylaxis in Different Countries
Many countries have introduced prophylactic regimens of vitamin K supplementation to prevent VKDB (23). The optimal dose, route, and frequency of administration of vitamin K, however, are still unclear. Oral and intramuscular (IM) regimens of vitamin K administration at birth have been proven effective in the prevention of classic VKDB (24). A single dose of IM vitamin K at birth can also prevent late VKDB. Between 1990 and February 2011, all infants in the Netherlands received a single oral dose of 1 mg vitamin K at birth, followed by a recommended daily oral supplementation of 25μg vitamin K between week 2 and 13 in breastfed infants (25). This regimen significantly decreased the incidence of late VKDB. In fact, in >80% of infants with biliary atresia (BA), severe late VKDB was the presenting symptoms.
The risk of VKDB in Dutch breastfed BA patients was 8 to 10 times higher than that of Danish BA patients, on either a weekly oral dose of 1 mg vitamin K or a single dose of 2 mg vitamin K at birth. Since March 2011, the prophylactic regimen was changed in the Netherlands; the recommended daily oral dose of 25μg vitamin K was increased to 150μg daily for all breastfed infants from week 2 to 13 of life. The single dose of 1 mg vitamin K at birth was maintained.
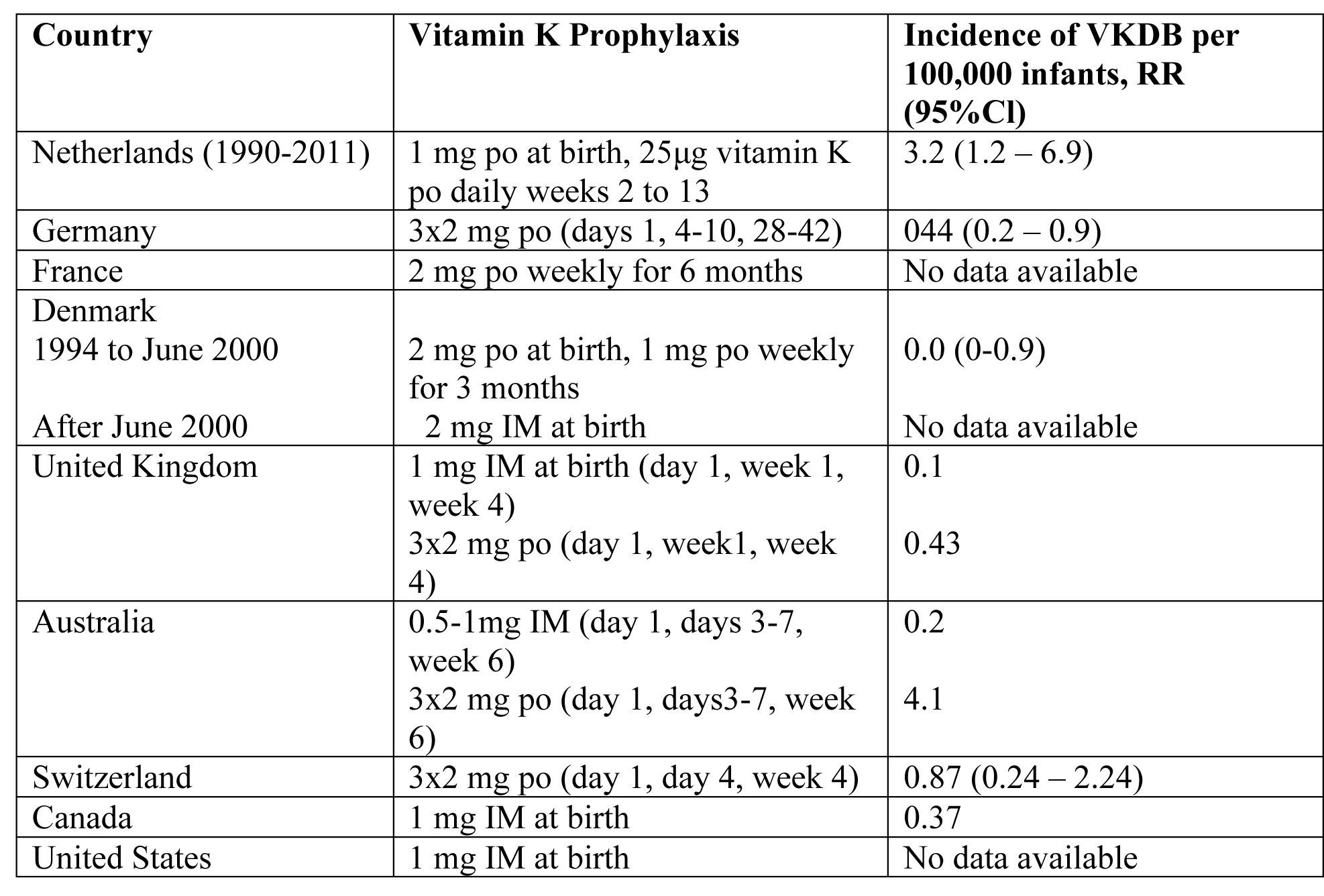
Table 2. VKDB: Vitamin K Deficiency Bleeding; Cl: confidence interval; po: by mouth; IM: intramuscular
Recommendation of American Academy of Pediatrics (AAP) in USA
Because parenteral vitamin K has been shown to prevent VKDB of the newborn and young infant and the risks of cancer have been unproven, the AAP recommends the following (8):
- Vitamin K1 should be given to all newborns as a single, intramuscular dose of 0.5 to 1 mg.
- Additional research should be conducted on the efficacy, safety, and bioavailability of oral formulations and optimal dosing regimens of vitamin K to prevent late VKDB.
- Healthcare professionals should promote awareness among families of the risks of late VKDB associated with inadequate vitamin K prophylaxis from current oral dosage regimes, particularly for newborns who are breastfed exclusively.
Prophylactic Vitamin K for Prevention of VKDB in Preterm Neonates
Preterm infants are potentially at greater risk of VKDB because of delayed feeding and subsequent delay in the colonization of their gastrointestinal system with vitamin K producing microflora, as well as immature hepatic and hemostatic function. This study reviewed the effect of vitamin K prophylaxis in the prevention of VKDB in preterm infants (26). The conclusion was preterm infants have low levels of vitamin K and develop detectable PIVKA proteins during the first week of life. Despite being at risk for VKDB, there are no studies comparing vitamin K versus non-treatment and few studies that address potential dosing strategies for effective treatment. Dosage studies suggest that we are currently giving doses of vitamin K to preterm infants that lead to supraphysiologic levels.
Because of current uncertainty, clinicians will have to extrapolate data from term infants to preterm infants. Since there is no available evidence that vitamin K is harmful or ineffective and since vitamin K is an inexpensive drug, it seems prudent to follow the recommendations of expert bodies and give vitamin K to preterm infants. However, further research on appropriate dose and route of administration is warranted.
The antenatal administration of vitamin K to mothers in preterm labor to prevent periventricular and intraventricular hemorrhage has been studied. In a systematic review of the literature, it is found to have no benefit giving antenatal vitamin K administration to preterm infants (27).
Prophylactic administration of vitamin K to prevent VKDB has been in practice for decades in USA, in both term and preterm infants. A single dose (1.0 mg) of intramuscular (IM) vitamin K after birth is effective in the prevention of classic VKDB in term infants. Either IM or oral (1.0 mg) vitamin K prophylaxis improves biochemical indices of coagulation status at one to seven days.
Preferred Vitamin K Preparations
There is no consensus as to the ideal formulation. Ensuring that newborns receive vitamin k is particularly critical in places where access to health care and blood products and transfusions is limited. Anecdotal evidence suggests that an investigation into the barriers preventing access to and widespread use of vitamin K prophylactic treatments in low-resource settings is warranted. Vitamin K prophylaxis can prevent VKDB.
The World Health Organization (WHO) recommends that newborns receive 1 mg of intramuscular (IM) injection of vitamin K at birth (28).
Reported product attributes of IM injection vs Oral formulation:
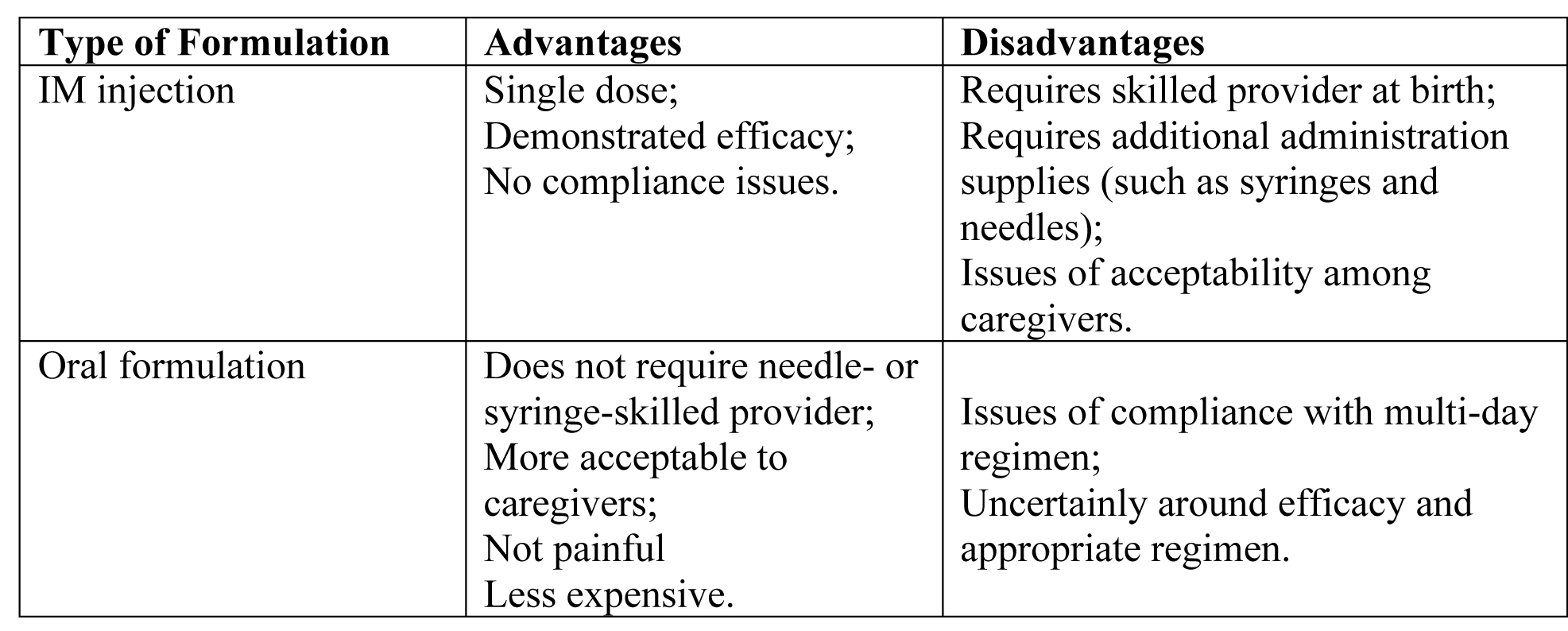
Table 3. Abbreviation: IM (intramuscular)
Implications for researchers
With significant benefits observed in the incidence of VKDB, policy-makers and other stakeholders are likely to give a high value to the routine administration of IM vitamin K (1 mg) at birth. The WHO also now recommends routine prophylaxis in all resource-restricted countries (29).
Controversies Concerning Vitamin K and the Newborn
Earlier concern regarding a possible causal association between parenteral vitamin K and childhood cancer has NOT been substantiated. Earlier studies using data from Great Britain attempted to show an association between intramuscular vitamin K administration in newborns and an increased incidence of childhood cancer (30). Using data from the National Registry of Childhood Tumors, they estimated the cumulative incidence of childhood leukemia. Three sources of data, including the estimates, provided rates of intramuscular vitamin K use over the same time frame. Their analyses failed to show a correlation between increased use of intramuscular vitamin K and the incidence of childhood leukemia.
The Vitamin K Ad Hoc Task Force of the American Academy of Pediatrics reviewed the reports of Golding et al, and other information regarding the US experience and concluded that there was no association between the intramuscular administration of vitamin K and childhood leukemia or other cancers (8).
Recent research on the pathogenesis of childhood leukemia additionally weakens the plausibility of a casual relationship between parenteral administration of vitamin K and cancer. Recent studies suggest a prenatal origin of childhood leukemia (31). This study found an acute lymphocytic leukemia-associated gene in 12 children with newly diagnosed acute lymphocytic leukemia and postulated that an in-utero chromosomal translocation event combined a postnatal promotional event results in clinical leukemia. Although intramuscular administration of vitamin K could conceivably be a postnatal promotional event, a genetic etiologic explanation further lessens the likelihood of a clinically significant relationship between intramuscular administration of vitamin K and leukemia.
Summary
Prevention of early vitamin K deficiency bleeding (VKDB) of the newborn, with onset at birth to 2 weeks of age (formerly known as classic hemorrhagic disease of the newborn), by oral or parenteral administration of vitamin K is accepted practice. In contrast, late VKDB, with onset from 2 to 12 weeks of age, is most effectively prevented by parenteral administration of vitamin K. Earlier concern regarding a possible casual association between parenteral vitamin K and childhood cancer has not ben substantiated. This revised statement presents updated recommendations for the use of vitamin K in the prevention of early and late VKDB. Given the high risk of mortality and morbidity in infants with late VKDB, it seems advisable to administer IM vitamin K prophylaxis to all neonates (term and/or preterm) at birth. Further studies should compare the efficacy and safety of multiple oral doses with IM vitamin K and also evaluate the optimal dose of vitamin K in preterm neonates.
References
- Van Winkel M, De Bruyne R, Van de Velde S, Van Biervliet S. Vitamin K, an update for the pediatricians. Eur J Pediatr 2009;168(2):127-134
- Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res 2012;56
- Ferland G. Vitamin K. In Erdman JW, MacDonald IA, Zeisel SH eds. Present knowledge in nutrition. 10th ed. Washington, D.C.: Wiley-Blackwell; 2012:230-247
- Suttie JW. Vitamin K. In: Coates PM, Betz JM, Blackman MR, et al. eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare 2010:851-860
- Ufer M. Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet 2005;44:1227-1246
- Centers of Disease Control and Prevention (CDC). Protect your baby from blees Fact Sheet; available @ https://www.cdc.gov/ncbddd/blooddisorders/documents/Vitamin-K-Provider-p.pdf Last accessed 10 July 2021
- Sutor AH, Kries R, Cornelissen EAM, et al. Vitamin K deficiency bleeding (VKDB) in infancy. Thromb Haemost 1999;81:456-461
- American Academy of Pediatrics (AAP); Controversies concerning vitamin K and the newborn; Committee on Fetus and Newborn. Pediatrics 2003; 112(1):191-192
- Witt M, Kvist N, Jorgensen MH, Hulscher JBF, et al. Prophylactic dosing of vitamin K to prevent bleeding. Pediatrics 2016; 137(5) e20154222
- Lippi G, Franchini M. Vitamin K in neonates: Facts and myths. Blood transfuse 2011;9:4-9
- Lagana AS, Vitale SG, Sapia F, Valenti G, et al. miRNA expression for early diagnosis of preeclampsia onset: Hope or hype? Matern Fetal Neonatal Med 2018;31:817-821
- Greer FR. Vitamin K the basics - What's the new? Early Hum Dev 2010;86:43-47
- Stafford DW. The vitamin K cycle. Thromb Haemost 2005;3:1873-1878
- Fukushima S, Tanaka T, Sato T, Shirakawa Y, et al. Prothrombin levels in newborn infants by the carinactivase-1 method. Semin Thromb Hemost 2002;28:539-544
- Chardot C. Biliary atresia. Orphanet J Rare Dis 2006;1:28
- Newman P, Shearer MJ. Vitamin K metabolism. Subcell Biochem 1998;30:455-488
- Cheng G, Tang CS, Wong EH, Cheng WW, et al. Common genetic variants regulating ADD3 gene expression alter biliary atresia risk. J Hepatol 2013;59:1285-1291
- Liu F, Zeng J, Zhu D, Zhang R, et al. Association of polymorphism in the VEGFA gene 3'-UTR+936T/C with susceptibility to biliary atresia in a Southern Chinese Han population. J Clin Lab Anal 2018;32:e22342
- Laughnan PM, McDougall PN. Epidemiology of late onset haemorrhagic disease: A pooled data analysis. J Paediatr Child Health 1993;29:177-181
- Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev 2009;23:49-59
- Sutor AH. New aspects of vitamin K prophylaxis. Semin Thrombo Hemost 2003;29:373-376
- Schreiner C, Suter D, Watzka M, et al. Genetic variants of the vitamin K dependent coagulation system and intraventricular hemorrhage in preterm infants. BMC Pdiatr 2014;14:219
- Health Council of the Netherlands. Advisory letter Vitamin K supplementation in infants. The Hague: Health Council of the Netherlands; 2010; Publication # 2010/11E. Available @ https://www.gezondheidsraad.nl/documenten/adviezen/2010/06/29/vitamine-k-suppletie-bij-zuigelingen Last accessed 10 August 2021
- Puckett RM, Offringa M. Prophylactic vitamin k for vitamin K deficiency bleeding in neonates. Cochrane Database Syst Rev 2000;(4): CD002776
- Cornelissen M, von Kries R, Loughnan P, Schubiger G. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K. Eur J Pediar 1997;156(2):126-130
- Ardell S, Offringa M, Ovelman C, Soll R. Prophylactic vitamin K for the prevention of vitamin K deficiency bleeding in preterm neonates. Cochrane Database Syst Rev 2018;2(2):CD008342
- Crowther CA, Crosby DD, Henderson-Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular hemorrhage. Cochrane Database of Systematic Reviews 2010; Issue 1: CD:000229
- Coffey PS, Gerth-Guyette E. Current perspective and practices of newborn vitamin K administration in low- and middle-income countries. Res Rep Neonatol 2018;8:45-51
- World Health Organization (WHO) Pocket Book of Hospital Care for Children: Guidelines for the management of common illness with limited resources. 2nd edition. WHO: Geneva, 2013
- Golding J, Paterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. Br J Cancer 1990;62:304-308
- American Academy of Pediatrics (AAP). Committee of Fetus and Newborn. Controversies concerning vitamin K and the newborn. AAP Publications Reaffirmed. Pediatrics 2015;135(2)e558
Published: 15 October 2021
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


