Ictère néonatal: partie I
Bulletin WHEC pratique et de directives cliniques de gestion pour les fournisseurs de soins de santé. Subvention à l'éducation fournie par la santé des femmes et de l'Education Center (WHEC).
Jaundice (hyperbilirubinemia) occurs in most newborns. Jaundice is benign most of the times in newborns, but because of potential toxicity of bilirubin, newborns must be monitored to identify those who might develop severe hyperbilirubinemia, and in rare cases, acute bilirubin encephalopathy or kernicterus. Based on a consensus of expert opinion and review of available evidence, universal pre-discharge bilirubin screening is recommended. This can be accomplished by measuring the total serum bilirubin level (ideally at the time of routine metabolic screening) or transcutaneous bilirubin level and plotting the result on an hour-specific nomogram to determine the risk of subsequent hyperbilirubinemia that will require treatment. If an infant is discharged before 24 hours postnatal age, the bilirubin should be rechecked within 48 hours.
The purpose of this guideline and review is to reduce the incidence of severe hyperbilirubinemia and bilirubin encephalopathy while minimizing the risks of unintended harm such as maternal anxiety, decreased breastfeeding, and unnecessary costs or treatment. Although kernicterus should almost be preventable, cases continue to occur. These guidelines provide a framework for the prevention and management of hyperbilirubinemia in newborn infants of 35 or more weeks of gestation. Kernicterus in detail is discussed in Neonatal Jaundice: Part II.
In every infant, the Women’s Health and Education Center (WHEC) recommends that clinicians:
- Promote and support successful breastfeeding;
- Perform a systematic assessment before discharge for the risk of severe bilirubinemia;
- Provide early and focused follow-up based on the risk assessment; and when indicated;
- Treat newborns with phototherapy or exchange transfusion to prevent the development of severe hyperbilirubinemia, and possibly bilirubin encephalopathy (kernicterus).
Background
The hyperbilirubinemia is often caused by an underlying hepatic or hematologic derangement but can also be a benign finding. Bilirubin is a normal by-product of hemoglobin metabolism. Plasma unconjugated (indirect) bilirubin is bound to albumin and taken up by hepatocytes, where it is conjugated with glucuronic acid. Conjugated (direct) bilirubin is secreted into bile and excreted. Hyperbilirubinemia can be caused by conditions leading to primarily unconjugated or primarily conjugated hyperbilirubinemia.
Jaundice, or yellowing of the skin, sclera, and mucus membranes by bilirubin is typically detectable clinically once serum bilirubin rises above 2.5 mg/dL and is often first detectable in the conjunctiva or under the tongue. Hyperbilirubinemia is dangerous in and of itself in newborns, where it can cross the blood brain barrier, deposit, and cause encephalopathy (kernicterus) at levels of 20-25 mg/dL.
In newborns, isolated hyperbilirubinemia is frequently associated with inborn error of bilirubin metabolism, and includes (1):
- Physiologic jaundice (generally resolves by day 10);
- Breast-milk jaundice (resolves if breast-milk is discontinued);
- Gilbert syndrome (benign);
- Dubin Johnson syndrome (benign);
- Crigler-Najjar syndrome, type I and type II. Crigler-Najjar syndrome can cause kernicterus, which must be treated.
Policies and Procedures for Hyperbilirubinemia
Each nursery should develop policies and procedures for hyperbilirubinemia screening. These policies should consider the following elements:
- Promotion and support of successful breastfeeding (2);
- Protocols for identification and evaluation of hyperbilirubinemia;
- Provision for measurement of the total serum bilirubin or transcutaneous bilirubin concentration in infants who are jaundiced in the first 24 hours;
- Recognition that visual estimation of the degree of jaundice can lead to errors, especially in darkly pigmented infants;
- Interpretation of all bilirubin levels according to the infant’s age in hours;
- Recognition that infants born less than 38 weeks of gestational age, especially those who are breastfed, are at higher risk of developing hyperbilirubinemia and require closer surveillance and monitoring;
- Performance of a systematic assessment on all infants before discharge for the risk of severe hyperbilirubinemia;
- Provision of written and verbal information to parents about newborn jaundice;
- Provision of appropriate follow-up based on the time of discharge and the risk assessment;
- Treatment, when indicated, with phototherapy or exchange transfusion.
Risk Factors for Development of Severe Hyperbilirubinemia
The best documented method for assessing the risk for hyperbilirubinemia is to measure the total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) and plot the results on a nomogram (see Fig. 1 below). A TSB level can be obtained at the time of the routine metabolic screen, thus obviating the need for an additional blood sample. Some authors have suggested that TSB measurement should be part of the routine screening of all newborns (3).
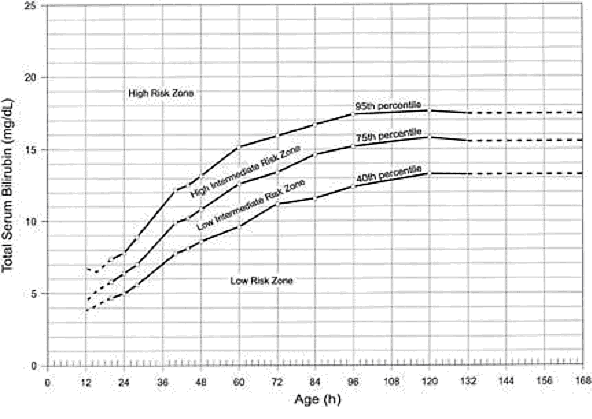
Figure 1: Nomogram for designation of risk in 2,840 well newborns at 36 or more weeks’ gestational age with birth weight of 2,000 g or more or 35 or more weeks’ gestational age and birth weight of 2,500 g or more based on the hour-specific serum bilirubin values. The serum bilirubin level was obtained before discharge, and the zone in which the value fell predicted the likelihood of a subsequent bilirubin level exceeding 95th percentile (high-risk zone).
Major risk factors
- Pre-discharge TSB or TcB level in the high-risk zone (4);
- Jaundice observed in the first 24 hour;
- Blood group incompatibility with positive direct antiglobulin test, other known hemolytic disease (e.g. G6PD deficiency), elevated end-tidal carbon monoxide concentration (ETCOc);
- Gestational age 35-36 weeks;
- Previous sibling received phototherapy;
- Cephalohematoma or significant bruising;
- Exclusive breastfeeding, particularly if nursing is not going well and weight loss is excessive;
- East Asian race.
Minor risk factors
- Pre-discharge TSB or TcB level in the high intermediate-risk zone;
- Gestational age 37-38 weeks;
- Jaundice observed before discharge;
- Previous sibling with jaundice;
- Macrosomic infant of a diabetic mother;
- Maternal age;
- Male gender.
Decreased risk (these factors are associated with decreased risk of significant jaundice, listed in decreasing importance) (5)
- TSB or TcB level in the low-risk zone;
- Gestational age >41 weeks;
- Exclusive bottle feeding;
- Black race;
- Discharge from hospital after 72 hours.
For clinical evaluation of jaundice, risk assessment, and treatment decisions, the discussion is divided into three groups:
- Term and late-preterm infants (35 weeks or more of gestational age);
- Preterm infants with a birth weight of 1,000 g or more;
- Extremely-low-birth-weights infants (less than 1,000 g birth weight).
- Inadequate intake: This condition is more common with a first-time breastfeeding mother, cesarean birth, maternal diabetes, or with a late preterm infant. Inadequate milk intake can result in excessive weight loss over the initial 7-14 days of age, marked hyperbilirubinemia, and rarely bilirubin encephalopathy or death. Caloric deprivation and increased enterohepatic circulation of bilirubin are more likely responsible for this result than dehydration (14). If inadequate milk intake persists, infants should be evaluated, rehydrated if needed, and supplemented with mother’s milk, if available, or infant formula.
- Breast milk jaundice: This condition is characterized by a persistence of physiologic jaundice beyond the first week of age. Breastfed infants commonly have serum bilirubin concentrations greater than 5 mg/dL (85.5 micromoles per liters) for several weeks after delivery (14). This persistent, mild unconjugated hyperbilirubinemia is caused by a variably identified factor in human milk that promotes an increase in intestinal absorption of bilirubin in combination with mutations of the uridine diphosphate-glucuronidase gene. Infants with jaundice that persists beyond 2 weeks of age should be monitored to ensure that the jaundice is not cholestatic in nature, the concentration of bilirubin is not increasing, and other pathologic causes for jaundice are not present.
- Phototherapy and assessment of mother-infant dyad during breastfeeding;
- Phototherapy with continued breastfeeding plus supplementation with mother’s expressed milk, if available, or infant formula. Supplementation with formula is recommended only if the mother’s milk supply is insufficient.
- Substitution of infant formula for breast milk for 24 hours. The bilirubin concentration should decrease at least 4 mg/dL. Mother’s milk production should be supported during this time, and breastfeeding resumed thereafter.
- Diarrhea;
- Interference with maternal-infant bonding;
- Intestinal hypermotility;
- Temperature instability.
- Increased risk of childhood asthma (odd ratio = 1.4) (32)
- Increased risk of type 1 diabetes mellitus (odd ratio = 3.79) (33)
- Phase 1 (first few days of life): Decreased alertness, hypotonia, and poor feeding are typical signs. Obviously, these are quite nonspecific and could easily be indicative of a multitude of neonatal abnormalities. A high index of suspicion of possible BIND at this stage that leads prompt intervention can halt the progression of the illness, significantly minimizing long-term sequelae. Of note, seizure is not typically associated with acute bilirubin encephalopathy. Among infants reported in the US kernicterus registry, the mean birth weight was 3,281 g (3).
- Phase 2 (variable onset and duration): Hypertonia of the extensor muscles is typical sign. Patients resent clinically with retrocollis (backward arching of the neck), opisthotonus (backward arching of the back), or both. Infants who progress to this phase develop long-term neurologic deficits.
- Phase 3 (infants aged >1week): Hypotonia is a typical sign.
- Dennery PA, Seidman DS, Stevenson DK. Neonatal Hyperbilirubinemia. NEJM 2001;344:581-590
- Ip S, Chung M, Kulig J, O'Brien R, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Pediatrics 2004;118:e130-153
- Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr 2002;140:396-403
- Stevenson DK, Fanaroff AA, Maisels MJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 2001;108:31-39
- American Academy of Pediatrics. Clinical Practice Guidelines; subcommittee on hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316 Available at: https://www.ncbi.nlm.nih.gov/pubmed/15231951 Retrieved on 19 October 2018
- Bhutani VK. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Committee on Fetus and Newborn, American Academy of Pediatrics. Pediatrics 2011;128:e1046-1052
- Carbonell X, Botet F, Figueras J, Riu-Godo A. Prediction of hyperbilirubinaemia in the healthy newborn. Acta Paediatr 2001;90:166-170
- Gottstein R, Cooke R. Systematic review of intravenous immunoglobulin in haemolytic disease in newborn of the newborn. Arch Dis Child Fetal Neonatal Ed 2003;88:F6-F10
- Ip S, Glicken S, Kulig J, Obrien R, et al. Management of Neonatal Hyperbilirubinemia. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; 2003. AHRQ Publication 03-E011
- Lamola AA, Bhutani VK, Du L, et al. Neonatal bilirubin binding capacity discerns neurological dysfunction. Peditr Res 2015;77:334-339
- Maisels MJ, Watchko JF, Bhutani VK, et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol 2012;32:660-664
- Hershcel M, Karrison t, Wen M, et al. Evaluation of the direct antiglobulin (Coombs') test for identifying newborns at risk for hemolysis as determined by end-tidal carbon monoxide concentration (ETCOc); and comparison of the Coombs' test with ETCOc for detecting significant jaundice. J Perinatol 2002;22:341-347
- Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks' gestation: an update with clarifications. Pediatrics 2009;124:1193-1198
- Stokowski LA. Still jaundiced after all these weeks: the breastfed neonate. Pediatrics 2014;134:e340-e345
- Bertini G, Dani C, Trochin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics 2001;107:3 Available at: http://pediatrics.aappublications.org/content/107/3/e41.full Retrieved on 20 January 2019
- Newman TB, Liljestrand P, Escobar GJ. Jaundice noted in the first 24 hours after birth in a managed care organization. Arch Pediatr Adolesc 2002;156:1244-1250
- Romero HM, Ringer C, Leu MG, et al. Neonatal jaundice: improved quality and cost savings after implementation of a standard pathways. Pediatrics 2018;141(3) e20161472
- Yasuda S, Itoh S, Isobe K, et al. New transcutaneous jaundice device with two optical paths. J Perinat Med 2003;31:81-88
- Bhutani VK, Maisels MJ, Stark AR, et al. Management of jaundice and prevention of severe neonatal hyperbilirubinemia in infants >or=35 weeks gestation. Neonatology 2008;94:63-67
- American Association of Blood Banks Technical Manual Committee. Perinatal issues in transfusion practice. In: Brecher M, ed. Technical Manual. Bethesda, MD: American Association of Blood Banks; 2002:497-515
- Gottstein R, Cooke R. Systematic review of intravenous immunoglobulin in haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2003;88:F6-F10
- Lo S, Doumas BT, Ashwood E. Performance of bilirubin determinations in US laboratories - revisited. Clin Chem 2004;50:190-194
- Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia, PA: W.B. Saunders; 2001
- Suresh G, Martin CL, Soll R. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev 2003;2:CD004207
- Kappas A, Drumond GS, Munson DP, et al. Sn-mesoporphyrin interdiction of severe hyperbilirubinemia in Jehovah's Witness newborns as an alternative to exchange transfusion. Pediatrics 2001;108:1374-1377
- Soorani-Lunsing I, Woltil H, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates really safe for the brain? Pediatr Res 2001;50:701-705
- Hsiao-Neng C, Meng-Luen L, Lon-Yen T. Exchange transfusion using peripheral vessels is safe and effective in newborn infants. Pediatrics 2008;122:e911-e916
- Brites D, Fernandes A. Bilirubin-induced neural impairment: a special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin Fetal Neonatal Med 2015;20(1):14-19
- Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin Fetal Neonatal Med 2015;20(1):20-25
- Olds C, Oghalai JS. Audiologic impairment associated with bilirubin-induced neurologic damage. Semin Fetal Neonatal Med 2015;20(1):42-46
- Slusher TM, Vreman HJ, Brearley AM, et al. Filtered sunlight versus intensive powered electric phototherapy in moderate to severe neonatal hyperbilirubinemia: a randomized controlled non-inferiority trial. The Lancet Global Health 2018;Vol. 6, No.10
- Aspberg S, Dahlquist G, Kahan T, et al. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol 2010;21(4 pt2):e733-e739
- Dahlquist G, Kallen B. Indications that phototherapy is a risk factor for insulin-dependent diabetes. Diabetes Care 2003;26(1):247-248
- Matichard E, Le Henanff A, Sanders A, et al. Blue light phototherapy of neonatal jaundice does not increase the risk of melanocytic nevus development. Arch Dermatol 2004;140(4):493-494
- Lee A, Folger LV, Mahmoodur R, et al. A novel icterometer for hyperbilirubinemia screening in low-resource settings. Pediatrics 2019;143(5):e20182039
Term and Late-Preterm Infants Without Added Risk Factors
There are few data on which to base treatment for term or late-preterm infants who do not have hemolytic disease or other risk factors and who have total bilirubin concentration of less than 20 mg/dL (342 micromoles per liter). Follow up data for apparently healthy term infants with bilirubin concentrations as high as 25 mg/dL (428 micromoles per liter) show no apparent neurologic sequelae (5),(6). However, historical data and subsequent studies have shown that a total serum bilirubin concentration greater than 30 mg/dL (513 micromoles per liter) carries a decidedly higher risk or permanent neurologic impairment (5),(6). The current guidelines for phototherapy and exchange transfusion for infants born at 35 weeks of gestation or greater are based on limited evidence and a consensus of expert opinions. \
Term and Late-Preterm Infants With Hemolysis and Other Risk Factors
A direct association between severe hemolysis and a rapid increase in unconjugated hyperbilirubinemia, bilirubin encephalopathy, and kernicterus has been demonstrated in infants with erythroblastosis fetalis. Survivors may manifest serious sequelae, including athetoid cerebral palsy, hearing loss, paralysis of upward gaze, and dentoalveolar dysplasia. Although no specific total serum bilirubin threshold for neurotoxicity has been established, clinical observations of term infants without hemolytic disease indicate that clinical bilirubin encephalopathy is unlikely at unconjugated bilirubin concentrations of less than 20 mg/dL (342 micromoles per liter) (7). However, total serum bilirubin concentration alone has been found to be an inadequate predictor of individual risk. Measures of free (unbound) bilirubin or reserve albumin binding capacity identify infants at risk more accurately, but these measures are not widely available.
The 95th percentile rate of increase in the first few days after birth is 0.25 mg/dL per hour (8). Any rate of increase in bilirubin concentration above this rate provides presumptive evidence of a hemolytic process and should prompt lowering the threshold for treatment by 3-5 mg/dL depending on severity. Visible jaundice during the first 24 hours after birth is also indicative of excessive hemolysis but it is not reliably seen.
Preterm Infants
Susceptibility to bilirubin toxicity as gestational age decreases. A recent report found that the average bilirubin binding capacity of preterm infants increase by 0.93 mg/dL for each week of gestation (9). Coincidently, albumin concentration increases by approximately 0.11 mg/dL per week of gestation. These data provide a rationale for lowering treatment threshold by approximately 1 mg/dL depending on severity. Visible jaundice during the first 24 hours after birth is also indicative of excessive hemolysis but it is not reliable.
Extremely-Low-Birth-Weight Infants
The neurodevelopment effects of aggressive phototherapy versus conservative phototherapy were compared in a large multicenter trial. Aggressive phototherapy was started in this study entry and continued for total bilirubin values exceeding 5 mg/dL during the first postnatal week and values greater than 7 mg/dL during the second week (10). Conservative phototherapy was started at 8 mg/dL for newborn infants with birth weights of 501-750 g and at 10 mg/dL for newborn infants with birth weights of 751-1,000 g (10),(11). Exchange transfusion was performed if phototherapy failed to bring the bilirubin below 13 mg/dL for the lower birth weight group and 15 mg/dL for the higher birth weight cohort. Aggressive phototherapy did not significantly reduce the rate of death or neurodevelopmental impairment at 18-22 months corrected age (52% versus 55%). Although aggressive phototherapy did significantly reduce the frequency of neurodevelopmental impairment in survivors (26% versus 30%), this reduction was offset by an increased mortality rate in infants weighing 501-750 g at birth (39% versus 34%) (11). Thus, when to initiate phototherapy in the extremely-low-birth-weight infant remains uncertain.
Laboratory Evaluation
Laboratory Evaluation of the jaundiced infants of 35 or more weeks’ gestation (5),(12)
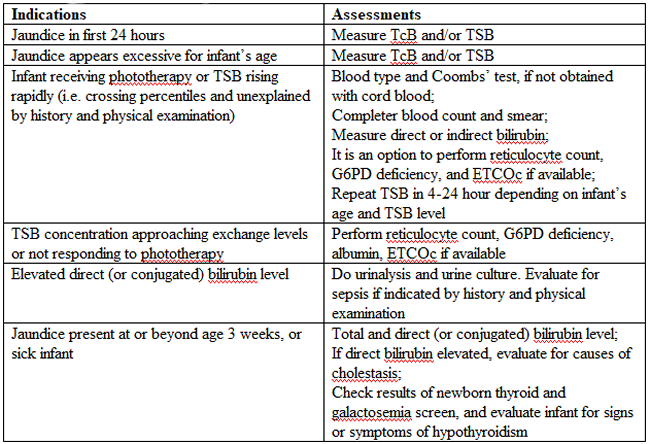
TSB(total serum bilirubin; TcB (transcutaneous bilirubin); ETCOc (end-tidal carbon monoxide concentration); G6PD (glucose-6-phosphate-dehydrogenase)
Breastfeeding and Hyperbilirubinemia
Frequent breastfeeding (8-12 breastfeedings every 24 hours) decreases the incidence of hyperbilirubinemia. Supplementing with water or dextrose-water does not ameliorate jaundice and cannot be recommended. Breastfeeding can significantly affect the incidence and duration of unconjugated hyperbilirubinemia compared with formula feeding in two ways (13):
Management: if the serum unconjugated bilirubin concentration in a breastfed, term healthy infant is increasing and exceeds 20 mg/dL (171 micromoles per liter), the physician has several options (15):
Dehydration and Hyperbilirubinemia
Most infants are readmitted to hospital with hyperbilirubinemia are not dehydrated, and phototherapy or exchange transfusion should not be delayed for rehydration. The infant should be sent directly to the service that can perform an exchange transfusion, if needed (16). Clinical assessment and the serum sodium concentration will indicate whether rehydration is needed or not.
Clinical Assessment
An evidence-based standard care pathway for neonatal jaundice can significantly improve multiple dimensions of value, including reductions in cost and length of stay (17). Newborn nurseries and newborn infant health care providers should have standard protocols to monitor all infants for the development of jaundice. Non-invasive transcutaneous bilirubin measurement devices can provide a valid estimate of the total serum bilirubin concentration of less than 15 mg/dL (257 micromoles per liter) in most infants (18). A transcutaneous or serum measurement of bilirubin concentration should be performed whenever jaundice is observed. The need and timing of a repeat transcutaneous bilirubin measurement or total serum bilirubin measurement will depend on the age of the infant and the progression of the hyperbilirubinemia. When serum bilirubin values exceed 15 mg/dL, measurement of serum albumin concentrations should be obtained.
Every newborn infant should undergo a risk assessment for developing hyperbilirubinemia before discharge to home. All infants should be examined by a qualified health care professional at 3-5 days postnatal age or within 48 hours of discharge to home to assess the infant's well-being and the presence or absence of jaundice.
Treatment
Treatment for neonatal hyperbilirubinemia includes phototherapy and exchange transfusion.
Phototherapy
Phototherapy is effective in reducing serum bilirubin concentrations in neonates with non-hemolytic jaundice. It is less effective in neonates with ABO and CDE (Rh) group hemolytic disease, reducing, but not eliminating, the need for exchange transfusions in these neonates (19). Exchange transfusion is the treatment of choice when the bilirubin concentration appears to pose an imminent threat to the health of the neonate.
There is no standardized method for delivering phototherapy. Commonly used phototherapy units contain daylight, cool white, blue or "special blue" fluorescent tubes. Other units use tungsten-halogen lamps in different configurations, either free-standing or as part of a radiant-warming device. Fiber optic systems have been developed that deliver high-intensity light via a fiber optic blanket.
The efficacy of phototherapy is influenced by the energy output (irradiance) in the blue spectrum (measured in microwatt per centimeter squared), the spectrum of light source, and the surface area of the neonate exposed to the light source, and the surface area of the neonate exposed to the light source. The irradiance of a unit should be monitored, and bulbs changed as needed to maintain maximum energy output. It is acceptable to interrupt phototherapy during feeding or brief parental visits. Intensive phototherapy can be achieved by use of blue lights, decreasing the distance of the source from the neonate and increasing the surface area exposed to the lights. The neonate’s temperature should be monitored frequently while phototherapy is being applied.
Although phototherapy has many biologic effects, it has no known lasting toxic effects in human neonate. Because experiments in animals have documented retinal damage from phototherapy, the neonate’s eyes should be covered with opaque patches during exposure to phototherapy light. Known potential complications from improper monitoring of eye-patch placement include exposure to high-energy light, malposition and obstruction of the nares, inadequate securing of the patch that allows lid opening and resultant corneal abrasion, and conjunctivitis from use without intermittent removal to assess the condition of the covered tissues.
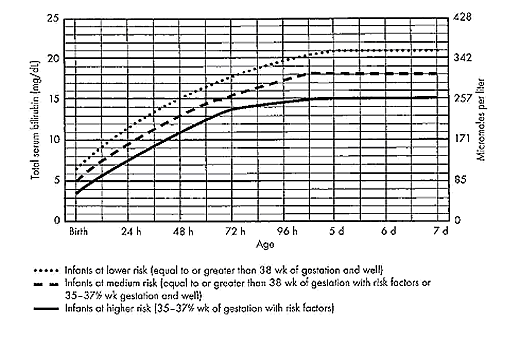
Figure 2: Guidelines for phototherapy in hospitalized infants at 35 weeks of gestation or older.
The guidelines shown in fig. 2, are based on limited evidence, and the levels shown above are approximations (5). These guidelines refer to the use of intensive phototherapy, which should be used when the total serum bilirubin level exceeds the line indicated for each category. Infants are designated as "higher risk" because of the potential negative effects of the conditions listed on albumin binding of bilirubin, the blood-brain barrier, and the susceptibility of the brain cells to damage by bilirubin. Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin. Risk factors are isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin less than 3 g/dL. For well infants 35-37 6/7 week of gestation total serum bilirubin levels can be adjusted for intervention around the medium risk line. it is an option to intervene at lower total serum bilirubin levels for infants closer to 35 weeks of gestation and at higher total serum bilirubin levels for those closer to 37 6/7 weeks of gestation. It is an option to provide conventional phototherapy in the hospital or at home with total serum bilirubin levels 2-3 mg/dL (35-50 micromoles per liter) below those shown, but home phototherapy should not be used in any infant with risk factors. Blue light phototherapy of neonatal jaundice does not increase the risk of melanocytic nevus development (34).
Adverse effects of neonatal phototherapy:
Short-term effects
Possible long-term effects
Exchange Transfusion
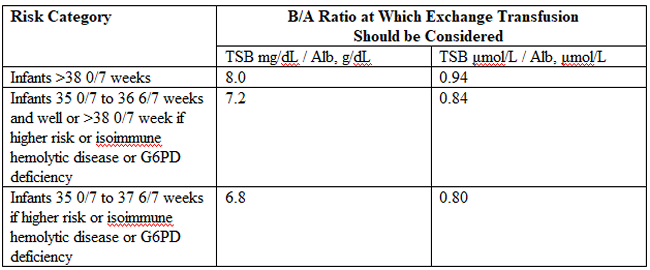
The guidelines in fig. 3 suggest levels represent a consensus in infants of the committee but are based on limited evidence, and the levels shown are approximations (5). During birth hospitalization, exchange transfusion is recommended if the total serum bilirubin level increases to these levels despite intensive phototherapy. For readmitted infants, if the total serum bilirubin (TSB) level is above the exchange level, repeat total serum bilirubin measurement every 2-3 hours and consider exchange if the total serum bilirubin level remains above the levels indicated after intensive phototherapy for 6 hours. The dashed lines for the first 24 hours indicate uncertainty because a wide range of clinical circumstances and a range of responses to phototherapy.
Immediate exchange transfusion is recommended if the infant shows signs of acute bilirubin encephalopathy (hypertonia, arching, retrocollis, opisthotonus, fever, or high-pitched cry) or if the total serum bilirubin level is equal to or greater than 5 mg/dL (85 micromoles per liter) above these lines. Risk factors are isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, and acidosis. Measure serum albumin level and calculate bilirubin/albumin (B/A) ratio. Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
If the infant is well and at 35-37 6/7 weeks of gestation (medium risk, total serum bilirubin levels for exchange) can be individualized based on actual gestational age.
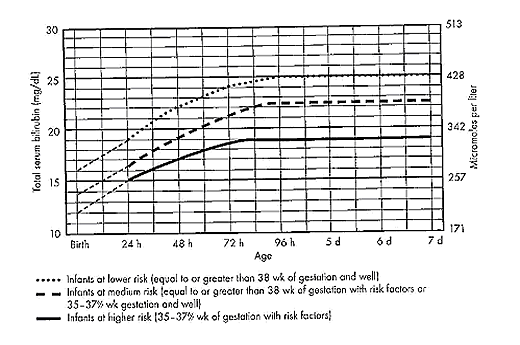
Figure 3: Guidelines for exchange transfusion in infants at 35 weeks of gestation or older.
The following B/A ratios can be used together with but in not in lieu of the TSB level as an additional factor in determining the need for exchange transfusion (20). If the TSB is at or approaching the exchange level, send blood for immediate type and cross match. Blood for exchange transfusion is modified whole blood (red cells and plasma) cross-matched against the mother and compatible with the infant (21).
Identification of Hemolysis: Because of their poor specificity and sensitivity, the standard laboratory tests for hemolysis are frequently helpful (22). However, end-tidal carbon monoxide, corrected for ambient carbon monoxide (ETCOc), levels can confirm the presence of absence of hemolysis, and measurement of ETCOc is the only clinical test that provides a direct measurement of the rate of heme catabolism, and the rate of bilirubin production (23). Thus, ETCOc may be helpful in determining the degree of surveillance needed and the timing of intervention. It is not yet known, however, how ETCOc measurements will affect management.
G6PD (glucose-6-phosphate-dehydrogenase)
It would be useful to develop an age-specific (by hour) nomogram for TSB in populations of newborns that differ regarding risk factors for hyperbilirubinemia. There is also an urgent need to improve to improve the precision and accuracy of the measurement of TSB in the clinical laboratory. Additional studies are also needed to develop and validate noninvasive (transcutaneous) measurements of serum bilirubin and to understand the factors that affect these measurements. These studies should also assess the cost-effectiveness and reducibility of TcB measurements in clinical practice (9).
Risks of Exchange Transfusion: Because exchange transfusion is now rarely performed, the risks of morbidity and mortality associated with the procedure are difficult to quantify. In addition, the complication rates listed below may not be generalizable to the current era if like most procedures, frequency of performance is an important determinant of risk. Death associated with exchange transfusion has been reported in approximately 3 in 1,000 procedures, although in otherwise well infants of 35 or more weeks' gestation, the risk is probably much lower (23). Significant morbidity (apnea, bradycardia, cyanosis, vasospasm, thrombosis, necrotizing enterocolitis) occurs in as many as 5% of exchange transfusions, and the risks associated with the use of blood products must always be considered (27). Hypoxic-ischemic encephalopathy and acquired immunodeficiency syndrome have occurred in otherwise healthy infants receiving exchange transfusions.
Pharmacologic Therapy
There is now evidence that hyperbilirubinemia can be effectively prevented or treated with tin-mesoporphyrin, a drug that inhibits the production of heme oxygenase (24). Tin-mesoporphyrin could find immediate application in preventing the need for exchange transfusion in infants who are not responding to phototherapy (25).
Acute Bilirubin Encephalopathy
Bilirubin-induced neurologic dysfunction (BIND) is the term applied to the spectrum of neurologic abnormalities associated with hyperbilirubinemia. It can be further divided into characteristic signs and symptoms that appear in the early stages (acute) and those that evolve over a prolonged period (chronic).
Acute Bilirubin Encephalopathy
The clinical features of this diagnosis have been well described and can be divided into 3 states. Of babies with BIND, approximately 55% - 65% present with these features, 20% - 30% may display some neurologic abnormalities, and approximately 15% have no neurologic signs (26).
The three stages are as follows:
BIND and classical kernicterus are clinical manifestations of moderate to severe hyperbilirubinemia whenever bilirubin levels exceed the capacity of the brain defensive mechanisms in preventing its entrance and cytotoxicity. In such circumstances and depending on the associated co-morbidities, bilirubin accumulation may lead to short- or long-term neurodevelopmental disabilities, which may include deficits in auditory, cognitive, and motor processing (28). Neuronal cell death, astrocytic reactivity, and microglia activation are part of the bilirubin-induced pathogenesis. Less understood is how abnormal growth and maturation of oligodendrocytes may impact on brain development, affecting the formation of myelin tracts. Based on in-vitro and in-vivo models, as well as in clinical cases presented in this series, authors propose the existence of impaired myelination by bilirubin with long-term sequelae, mainly in preterm infants (28).
Movement Disorders due to Bilirubin Toxicity
Advances in the care of neonatal hyperbilirubinemia have led to a decreased incidence of kernicterus. However, neonatal exposure to high levels of bilirubin continues to cause severe motor symptoms and cerebral palsy (CP). Exposure to moderate levels of unconjugated bilirubin may also cause damage to the developing central nervous system, specifically the basal ganglia and cerebellum. Brain lesions identified using magnetic resonance imaging (MRI) following extreme hyperbilirubinemia have been linked to dyskinetic CP (29). Newer imaging techniques, such as diffusion tensor imaging or single-photon emission computed tomography (CT), allow quantification of more subtle movement disorders.
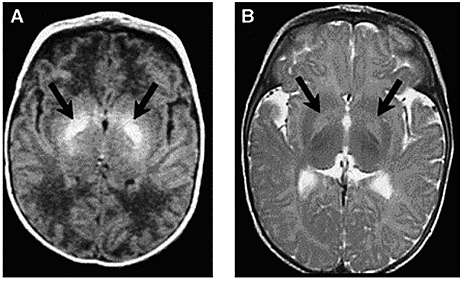
Figure 4: Axial MRI of bilateral hyperintense lesions in the globus pallidus in axial projections (arrows).
(A) T1-weighted axial image of a six-day-old, 37-week gestation boy with peak bilirubin of 34.6 mg/dL. At age 7 years, this child was highly intelligent, but moderately to severely disabled with dystonic, athetoid kernicteric cerebral palsy; he ambulates with a walker.
(B) Axial T2-weighted MRI of a 2-year old who has classic dystonic kernicterus. Note the increased intensity of the globus pallidus bilaterally (shown with arrows and dotted line on right side only). There were no abnormalities noted in brainstem or cerebellum.
New categories of bilirubin-induced neurologic dysfunction, characterized by subtle encephalopathy following moderate hyperbilirubinemia, have been implicated in long-term motor function. Further research is needed to identify subtle impairments resulting from moderate-severe neonatal hyperbilirubinemia, to understand the influence of perinatal risk factors on bilirubin toxicity, and to develop neuroprotective treatment strategies to prevent movement disorders due to bilirubin toxicity.
Audiologic Impairment associated with Bilirubin Toxicity
Hyperbilirubinemia occurs commonly in neonates and is usually mild and transient, with no long-lasting sequelae. However, bilirubin-induced neurologic damage may occur in some infants. The auditory pathway is the most sensitive part of the central nervous system to bilirubin-induced toxicity, and permanent sequelae may result from only moderately elevated total serum/plasma bilirubin levels. The damage to the auditory system occurs primarily within the brainstem and cranial nerve VIII, and manifests clinically as auditory neuropathy spectrum disorder (30).
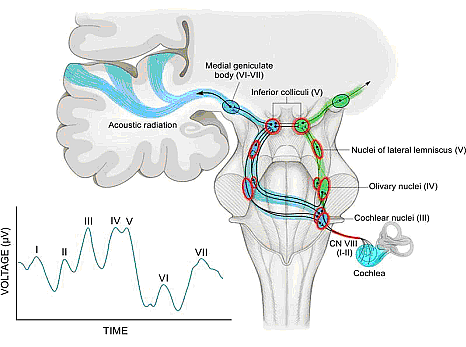
Figure 5: The auditory pathway and normal auditory brainstem response (ABR). The ipsilateral (green) and contralateral (blue) auditory pathways are shown, with structures that are known to be affected by hyperbilirubinemia highlighted in red. Roman numerals in parenthesis indicate corresponding waves in the normal human ABR (inset).
Sunlight Phototherapy for Neonatal Jaundice in Low- and Moderate-Income Countries
Kernicterus resulting from severe hyperbilirubinemia is a leading cause of preventable deaths in low- and middle-income countries, partly because high-quality intensive phototherapy is unavailable. Sunlight is available in abundance in most low- and middle-income countries but is grossly underused. Many studies have shown clear results that filtered-sunlight phototherapy (FSPT) can be as efficacious and safe as conventional intensive electric phototherapy (IEPT) for the treatment of moderate to severe neonatal hyperbilirubinemia.
In this prospective, randomized controlled non-inferiority trial in Ogbomosho, Nigeria – a simulated rural setting, showed promising results (31). Near-term or term infants aged 14 days or younger who were of 35 weeks or more gestation and with total serum bilirubin concentrations at or above the recommended age-dependent treatment levels of high-risk neonates were randomly assigned (1:1) to either FSPT or IEPT. Randomization was computer generated, and neither clinicians nor the parents or guardians of participants were masked to group allocation. The conclusion was FSPT is safe and no less efficacious than IEPT for treatment of moderate to severe neonatal hyperbilirubinemia in near-term infants.
The Bili-ruler: a low-cost icterometer in low-resource settings
The Bili-ruler, a novel low-cost icterometer, can be used to classify different clinical thresholds of hyperbilirubinemia with high diagnostic accuracy. The majority of bilirubin-related encephalopathy and mortality is concentrated in low-income countries and remote rural areas, where accurate and low-cost methods for jaundice management by frontline health workers in low-resource settings are needed. The Bili-ruler may enable health workers to more rapidly and accurately identify infants with hyperbilirubinemia at peripheral levels of the health care system or in communities and provide them with early referral and/or timely treatment with phototherapy.
This study (35) aimed to determine 1) the validity of the novel icterometer (Bili-ruler) to detect clinically significant threshold of hyperbilirubinemia, and 2) assessing reliability of icterometer scoring. The conclusion was the Bili-ruler is a low-cost and non-invasive tool with high diagnostic accuracy for neonatal jaundice screening. This device may be used in improve referrals from community or peripheral health centers to higher-level facilities with capacities for bilirubin testing and/or phototherapy.
Kernicterus
Neonatal Jaundice: Part II
www.womenshealthsection.com/content/obsnc/obsnc006.php3
Summary
Before discharge, every newborn should be assessed for the risk of developing severe hyperbilirubinemia, and all nurseries should establish protocols for assessing this risk. Such assessment is particularly important in infants who are discharged before the age of 72 hours. Although breastfeeding per se does not seem related to the increased frequency of neonatal jaundice in the first days of life, but rather to the higher bilirubin level in a very small sub-population of infants with jaundice. These infants, when starved and/or dehydrated, could probably be at the higher risk of bilirubin encephalopathy, especially after discharge from the hospital when careful follow-up is lacking. Among other well-known conditions favoring neonatal jaundice, it must be emphasized that infants born by vacuum extraction are at risk of exaggerated neonatal jaundice.
References
Publié: 14 May 2019
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


