Epidural Analgesia Failures: The Technique Review
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
The goal of epidural analgesia is to provide satisfactory pain control for labor with the lowest dose of analgesic drugs needed to minimize motor blockage and simultaneously reduce the potential side effects of epidural analgesia during the course of labor. Epidural analgesia offers the most effective form of pain relief and is used by most women in the United States. The overwhelming majority of epidural catheters placed for labor provide satisfactory analgesia. There are, however, times when the catheter is not at the correct site within the epidural space, the patient's neuraxial anatomy is problematic, or a patient's labor progresses more quickly than expected by the anesthesiologist, and the epidural block does not set up on time. Migration of the epidural catheter is given as the main cause of cessation after a successful block. Patient-controlled epidural analgesia provides pain control similar to that of standard epidural analgesia. Patient satisfaction with all epidural techniques is high and is not significantly improved with patient-controlled epidural analgesia. The decision of when to place epidural analgesia should be made individually with each patient, with other factors, such as parity, taken into consideration.
The purpose of this document is to explore the basics of neuraxial labor analgesia, the causes of its failure, and the strategies anesthesiologists employ to rescue poorly functioning catheters. Uterine contractions and cervical dilatation result in visceral pain (T-10 through L-1). As labor progresses, the descent of the fetal head and subsequent pressure on the pelvic floor, vagina, and perineum generate somatic pain transmitted by the pudendal nerve (S2-S4). Ideally, methods of obstetric pain relief will ameliorate both sources of pain in the patient in labor. Patients with a history of back surgery, especially those who have had spinal instrumentation and fusion to correct scoliosis, have increased rates of epidural failure. Fortunately, in patients with a history of back surgery, epidural analgesia is often successful.
Incidence of Epidural Failure:
The overall success rate of epidural analgesia in labor is approximately 98% to 99%. The most comprehensive review of obstetric neuraxial failures shown in a retrospective analysis of 19,259 deliveries that demonstrated an overall failure rate is of 12%. Of the neuraxial techniques, 46% became functional with simple manipulations. Overall, 7.1% of patients receiving neuraxial analgesia had their catheters replaced and 1.9% had multiple replacements. In the end, 98.8% of patients reported adequate labor analgesia (1). The epidural space is filled with fat, connective tissue, and an extensive venous plexus. It behaves less like a collapsed Penrose drain and more like a container filled with sand and variously sized pebbles, such that drugs must traverse a maze of obstacles to reach the nerves. The anatomic labyrinth may result in the initial placement of the catheter tip in an unfavorable micro-environment. When the initial epidural placement and infusion does not result in an adequate block, anesthesiologists often perform two different maneuvers to salvage the catheter: partial withdrawal of the catheter or infusion of additional medication. Both of these maneuvers have proven successful and are employed if the epidural failure is from a unilateral or asymmetric block that does not extend to sacral dermatomes (sacral sparing).
Neuraxial Anatomy and Epidural Technique:
The epidural space is a potential space in the neuraxis deep (anterior) to the ligamentum flavum and shallow (posterior) to the dura mater. Anesthesiologists use a loss-of-resistance technique to determine when the tip of a hollow epidural needle is at site just anterior to the thick ligamentum flavum and in the epidural space. When the tip of the epidural needle lies within a ligament, injection of air or saline is difficult. Upon entry into the epidural space, this resistance to injection is lost. An epidural catheter is then threaded 3 to 5 cm into the epidural space. After the catheter is at the site in the epidural space, epidural drugs are injected and slowly soak into the nerve roots of the low thoracic, lumbar, and sacral spine, providing analgesia within about 20 minutes (2). In patients undergoing cesarean delivery under epidural anesthesia, patient-controlled epidural analgesia for the first 24 hours after the surgery, is a reasonable choice. This strategy minimizes the dosage of maternally administered opioids and maternal sedation compared with intravenously administered opioids (patient-controlled administration) and uses the preexisting catheter. Although patient-controlled epidural analgesia using a combination of opioid and local anesthesia reduces the cumulative opioid dosage, it results in increased motor weakness, which may inhibit patient mobilization (3). Even low concentrations of local anesthesia result in significant motor weakness and can make ambulation difficult in up to 43% of patients. Consideration should be given to removing the epidural catheter after 24 hours to reduce side effects, such as urinary retention, pruritus and infection risk, as well as to minimize the costs. n
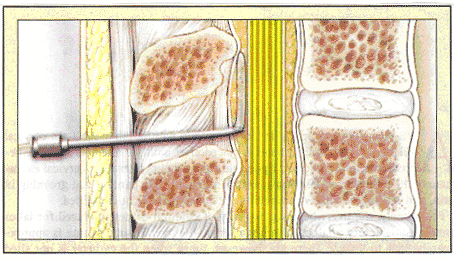
Catheter Placement into Epidural Space
In the combined spinal epidural technique (CSE), after the epidural needle is properly at the site, a small-gauged spinal needle is passed through the bore of the epidural needle, through dura mater, and into the intrathecal space. Flow of cerebral spina fluid (CSF) back through the spinal needle confirms its location. After drug is deposited into CSF, the spinal needle is removed, and an epidural catheter is threaded through an epidural needle into the epidural space (2). The intrathecal drug provides a faster onset of analgesia than an epidural alone -- usually within 5 minutes. The use of newer "atraumatic" spinal needles is associated with a dramatically decreased risk of spinal headache. The spinal component of combined spinal epidural (CSE) may be an intrathecal narcotic plus a small amount of a local anesthetic. Failure of the spinal component occurs at a rate of 4% with CSE, but the block can be supplemented with the epidural catheter (11). n
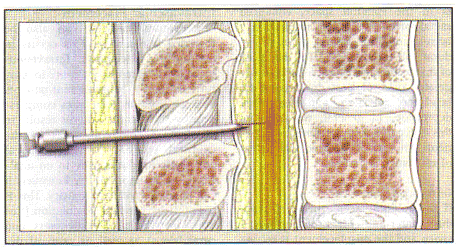
Combined Spinal Epidural (CSE) Technique
Both opioids and local anesthetics are typically used in neuraxial labor analgesia. Fentanyl is generally the opioid of choice because its lipophilicity results in excellent immediate mu-opioid receptor agonism in the neuraxis and minimal central side effects. Bupivacaine is the local anesthetic of choice because, in comparison with other local anesthetics, it preferentially blocks sensory over motor nerves, preserving lower extremity and abdominal muscle strength. Volume and drug concentration need to be considered in epidural dosing, whereas the drug's baricity (density compared to CSF) within the CSF and overall dose (milligrams of drug) need to be considered in the intrathecal space. The dose required in the epidural versus the intrathecal space differs by approximately a factor of 10. An epidural catheter unknowingly placed in the intrathecal space could result in a massive overdose, potentially resulting in a "high spinal". When this occurs, respiratory distress, hypotension, and fetal distress can ensue. Therefore, anesthesiologists take care and time in dosing, even if women are in significant pain. As a result, parturients may progress rapidly through labor or even deliver prior to the onset of effective analgesia.
Causes of Epidural Analgesia Failure:
In general, a continuous and even distribution of local anesthetic within the epidural space bathing the appropriate nerve roots leads to successful labor analgesia. The causes of neuraxial labor analgesia failure include inadequate initial epidural needle placement, suboptimal catheter at site in epidural space upon threading, catheter migration within the epidural space during labor, problematic neuraxial anatomy of the parturient, or an unpredictably fast labor. Further considerations that may contribute to epidural failure include the type of catheter used (multi-orifice vs single-orifice vs newer single orifice, soft-tipped, wire-reinforced catheters) (4), the distance the catheter is threaded into the epidural space, the experience of the individual placing the epidural, whether a CSE technique is performed, and differences in patient expectations. A debatable consideration is whether air or saline is used in the loss-of-resistance technique (5). The anesthesiologist may or may not detect misplacement of the epidural needle at the time of placement. Aside from the epidural space, the needle may be in the subcutaneous or other paravertebral tissue, past the dura mater and into the intrathecal space, in the subdural space (between the dura and arachnoid), or in an epidural vein. An intrathecal or intravenous placement will be heralded by the flow of CSF or blood from the hub of the needle. This may not always occur, however, and thus the standard of care is aspiration on the catheter after it is threaded and slow dosing of the medication divided into small aliquots. During administration of each epidural dose, the anesthesiologist watches for signs of an inappropriately dense or high level of anesthesia (intrathecal placement) or symptoms of systemic local anesthetic toxicity (intravascular placement).
Subcutaneous Placement: A false loss-of-resistance, in which the tip of epidural needle lies within subcutaneous tissue, is the likely cause of most complete epidural failures (ie, no block) (5). Local anesthetic and the low concentration of opioid in epidural infusions provide little to no analgesia for the parturient when deposited in subcutaneous tissue. Such a failure is usually detected in less than 30 minutes after the initial placement when no level of sensory blockade can be detected in the legs or lower abdomen and the patient remains in pain. However, in some cases the parturient may report analgesic relief that the anesthesiologist may inappropriately attribute to a successful epidural. This may delay the replacement of the misplaced epidural, and may occur if concentrations transiently decrease (eg, from pre-epidural fluid loading or discontinuation of oxytocin during placement), if systemic opioid absorption causes analgesic effects, or even if the placement has a placebo effect for the parturient. Some anesthesiologists perform a CSE to assure that the needle is in the epidural space. By placing a spinal needle through the epidural needle and obtaining CSF at the time of placement, they assure themselves that the dura mater and intrathecal space is just beyond the tip of the epidural needle, which must be, therefore, in the epidural space. At this time anesthesiologist may inject an intrathecal medication such as an opioid and/or local anesthetic. Advocates for this technique cite the fact that epidurals placed after a CSE technique have been found to be more successful than conventional epidurals (1). Opponents may cite anecdotal evidence of a spinal dose masking an inadequately placed epidural catheter that subsequently fails upon bolusing for urgent cesarean delivery, risking an unexpected general anesthetic. An alternative method is to perform a dural puncture without drug administration, solely to confirm proper position of the epidural needle. Aside from the confirmation that the needle is in the epidural space, enhanced sacral spread of the block results, likely because of drug passing through the dural hole into the CSF (5). The downside of the technique includes theoretically greater risks of post-dural puncture headache, infection, and respiratory depression. In general, it is our recommendation, the CSE confirmation technique in patients who are obese or in whom loss of resistance is unclear.
Intrathecal Placement: If the epidural needle penetrates the dura mater and enters the intrathecal space and the misplacement is recognized by the free flow of CSF from the needle, the catheter may be threaded into the spinal space and may still be safely used for labor analgesia or surgical anesthesia. Such spinal catheters are usually not used intentionally due to a high risk (>50%) of a post-dural puncture headache, as well as theoretically increased risk of infection.
Subdural Placement: Rarely, catheters can be at the site in the subdural space. This is a potential space between the dura mater and the pia-arachnoid membrane. If a catheter is threaded into this location and a large dose of medication intended for the epidural space is injected here, an unusually patchy and potentially dangerous block can occur that will likely result in inadequate analgesia as well as the signs and symptoms of a high spinal. Because no CSF will be aspirated and the onset of the block is slow, it is often difficult to detect. Recent investigations have suggested this phenomenon may occur more frequently than previously suspected (6).
Intravascular Placement: The epidural catheter can also be threaded into a vein. This occurs in 5% to 7% of placements (1). Intravascular placement is more common in parturients than in non-pregnant patients because inferior vena cava compression by the enlarged uterus results in dilatation of collateral veins in the epidural space. Intravenous local anesthetic produces little to no analgesia. More importantly, however, it can result in systemic toxicity. This is manifested by cerebral stimulation (tinnitus, metallic taste, restlessness, and convulsions) as well as cerebral depression (unconsciousness), cardiovascular toxicity (bradycardia, vasodilation and hypotension, and ventricular fibrillation), and uterine vasoconstriction and hypertonus. Hemodynamic and respiration support are the mainstays of treatment, but recent advances in aborting cardiac arrest with lipid emulsion therapy are promising (7).
Asymmetric Block: It can either be completely unilateral or can manifest as windows in an otherwise complete block, in which one or more dermatomes are spared. Despite apparently proper placement, approximately 5% to 8% of epidural blocks may provide incomplete analgesia of this sort (1). Although the cause of any particular failure is usually unknown, it is generally believed that either an anatomic barrier to free flow of local anesthetic or unfavorable positioning of the tip of the catheter is responsible for asymmetric block. Multiple and extensive studies evaluating epidural anatomy have been done including injecting cadaveric epidural spaces with resin, cadaveric epiduroscopy, computed tomography, and anatomic dissection with cryomicrotome section. The presence of a dorsal median connective tissue band (DMCTB) in some individuals has also been described but is generally thought to be rare and incomplete when present. This DMCTB has been attributed to be an artifact of how the epidural space was studied (8). Radiologic studies have shown that when a catheter is threaded beyond the tip of the needle in epidural space it rarely stays in the midline, and it may also be directed caudally rather than the preferred cranial direction. Anesthesiologists compensate for this by providing a large volume of a dilute solution of a local anesthetic and opioid in order to ensure spread of medication to both sides of the epidural space as well as up and down the spine. In addition, studies show that when catheters are threaded no more than 5 cm into the epidural space, fewer insufficient blocks occur. Perhaps this is because these catheters are less likely to be at the site laterally or, even worse, to exit the epidural space along the course of a nerve root. When these events occur, the spread of the local anesthetic may be limited to either a specific side or a specific dermatome (8). This provides additional rationale for partially withdrawing an epidural catheter producing an asymmetric block.
Sacral Sparing: Even with a large volume of dilute local anesthetic and with an appropriate length of catheter in the epidural space, analgesia may still be inadequate. Typically, anesthesiologists place epidurals in the inner-spaces between the L2-3, L3-4, or L4-5 vertebral bodies. For the first stage of labor, the pain is largely visceral and carried by the T10 through L1 nerve roots, which innervate the cervix, uterus, and upper portion of the vagina. For the second stage of labor, the pain is somatic and carried by the S2-S4 nerve roots, innervating the perineum. Not only is the pain of second-stage of labor more intense, but these nerve roots are further from the tip of the epidural catheter, larger in diameter, and surrounded by thicker dura mater, and therefore more difficult for local anesthetic to penetrate. Moreover, epidural infusions tend to traverse upward within the epidural space more readily than downward. Therefore, the local anesthetic from even a well-placed epidural catheter may fail to adequately bathe and penetrate the sacral nerve roots and provide relief for the more intense pain of second-stage labor. This sacral sparing phenomenon leads some anesthesiologists to prefer a CSE technique when delivery is imminent. The intrathecal local anesthetic directly bathes the sacral nerve roots in the cauda equine and the opioid directly binds the mu-opioid receptors of the substantia gelatinosa in the spinal cord. The onset of relief is faster and sacral coverage is superior (9).
Migration of Epidural Catheter: An epidural analgesia that works well initially may not continue to work well throughout the entire labor and delivery. The position of epidural catheters is not static, and catheters may migrate completely out of the epidural space (resulting in cessation of analgesia), laterally (resulting in a unilateral block), or even intrathecally or intravascularly (resulting in toxicity). The migration of epidural catheters is not rare; in large retrospective series, 6.8% of patients with initially adequate blocks subsequently developed insufficient analgesia (1).
Prior Back Surgery and Chronic Back Pain: Patients with a history of chronic low back pain have increased rates of failure. In patients with unilateral back pain or sciatica, the affected nerve roots become blocked 10 to 70 minutes later than the contralateral side purportedly because the local anesthetic has difficulty diffusing into the injured area. Likewise, disk herniation may cause scarring and epidural adhesions that slow the diffusion of local anesthetic past the injured area or inhibit it altogether (10). Uncorrected scoliosis can lead to difficulty in finding the epidural space, poor spread of local anesthetic, and an increase in dural puncture and other complications. Patients with a history of back surgery, especially those who have had spinal instrumentation and fusion to correct scoliosis, also have increased rates of epidural failure. In patients who have had corrective scoliosis surgery, the epidural space may be impossible to find because of instrumentation, bone graft material, or scar tissue in the area of the fusion and degenerative changes in the area below the fusion. If epidural placement is possible, patchy spread of local anesthetic may occur from intraoperative trauma to the ligamentum flavum leading to scarring and obliteration of the epidural space. These patients also may have a high rate of unintentional dural puncture with the subsequent inability to perform a blood patch for fear of repeat dural puncture or infection of their spinal hardware. Some anesthesiologists consider epidural analgesia to be relatively contraindicated in these patients. Success has been reported in 91% of patients who had previous back surgery, compared with 98.7% of patients who had not in a series of 1381 non-pregnant patients (8). The authors attributed this result to the routine use of large volumes of epidural anesthetic and operator skill.
Summary:
Anatomic, functional, technical, and random variations may seem to conspire against the anesthesiologists in the attempt to provide epidural analgesia to the parturient. Fortunately, with proper technique nearly 99% of women can expect satisfactory analgesia. Large volumes of diluted anesthetic, manipulation of the catheter, use of the CSE technique, and willingness to replace a poorly functioning catheter can rescue the overwhelming majority of imperfect epidural analgesics in labor. The causes of neuraxial labor analgesia failure include inadequate initial epidural needle placement, suboptimal catheter at site in epidural space upon threading, catheter migration within epidural space during labor, problematic neuraxial anatomy of the parturient, or an unpredictably fast labor. A false loss-of-resistance, in which the tip of the epidural needle lies within subcutaneous tissue, is the likely cause of the most complete epidural failure. Intravascular placement of the epidural catheter is more common in parturients. Intravascular local anesthetic produces little or no analgesia and can result in systemic toxicity.
The position of epidural catheters is not static, and catheters may migrate completely out of the epidural space (resulting in cessation of analgesia), laterally (resulting in a unilateral block), or even intrathecally or intravascularly (resulting in toxicity). Patients with a history of chronic low back pain or back surgery have increased rates of epidural failure. Combine spinal epidural (CSE) analgesia offers the rapid onset of spinal analgesia combined with the ability to use the epidural catheter to prolong the duration of analgesia with a continuous infusion for labor, to convert to anesthesia for cesarean delivery, or to provide post-cesarean delivery pain control (11). This method of obstetric analgesia is increasing in popularity, especially with the advent of needle-through-needle techniques that eliminates the need for more than one skin puncture.
References:
- Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth 2004;13:227-233
- Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med 2003;348:319-332
- ACOG Practice Bulletin. Obstetric analgesia and anesthesia. Number 36, July 2002
- Jaime F, Mandell GL, Vallejo MC et al. Uniport soft-tip, open-ended catheters versus multiport firm-tipped close-ended catheters for epidural labor analgesia: a quality assurance study. J Clin Anesth 2000;12:89-93
- Arendt K, Segal S. An effectiveness study of air versus saline is used for identification of the epidural space by loss of resistance. Anesth Analg 2008;106:A-1-A-221. Abstract A-104
- Collier CB. Accidental subdural injection during attempted lumbar epidural block may present as a failed or inadequate block: radiographic evidence. Reg Anesth Pain Med 2004;29:45-51
- Corman SL, Skledar SJ. Use of lipid emulsion to reverse local anesthetic-induced toxicity. Ann Pharmacother 2007;41:1873-1877
- Arendt K, Segal S. Why epidural do not always work. Rev Obstet Gynecol 2008;1(2):49-55
- Bucklin BA, Hawkins JL, Anderson JR et al. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology 2005;103:645-653
- Weinberg GL. Current concepts in resuscitation of patients with local anesthetic cardiac toxicity. Reg Anesth Pain Med 2002;27:568-575
- Abrao KC, Francisco RPV, Miyadahira S et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia. Obstet Gynecol 2009;113:41-47
Published: 30 September 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


