Профилактика стрептококковой инфекции группы B у новорожденных: перинатальное ведениеWHEC практики Бюллетень и клинических управления Руководство для медицинских работников. Образования гранта, предоставленного здоровья женщин-образовательный центр (WHEC). Group B streptococcus (GBS) is the leading cause of newborn infection. It is a common commensal in the gut of humans and in the lower genital tract in women, remains an important cause of neonatal mortality and morbidity. The incidence of early-onset disease has fallen markedly in countries that test women for carriage at 35 – 37 weeks of pregnancy and then offer intrapartum prophylaxis with penicillin during labor. Countries that do not test, but instead employ a risk factor approach, have not seen a similar fall. There are concerns about the effect on the neonatal microbiome of widespread use of antibiotic prophylaxis during labor, but so far the effects seem minor and temporary. Universal screening and antibiotic prophylaxis of all GBS carriers result in increased antibiotic exposure in most of the populations, which might carry its own risks. Therefore, patients should be involved in decisions on whether to be screened or not, is based on identification of risks and benefits. Vaccination against GBS would be acceptable to most women and GBS vaccines are in the early stages of development. The purpose of this document is to identify limitations of current strategies for antepartum and intrapartum prophylaxis of neonatal early-onset group B streptococcal (GBS) infection. Better strategies are needed to assure timely administration when prophylaxis is indicated. Neonatal sepsis continues to cause a significant proportion of perinatal mortality and long-term morbidity in the term and preterm infant population. The most common single organism that causes early-onset neonatal sepsis is the group B streptococcus (GBS or Streptococcus agalactiae) (1). The present guidelines are designed to lower the risk of GBS early-onset-disease, which is the most common cause of early-onset neonatal sepsis. What is Group B Streptococcus (GBS)?Antony van Leeuwenhoek (1632 - 1723) was the first to identify microscopic one-celled organisms, which he called 'animalcules.' He described bacteria of the genus Selenomonas (crescent shaped bacteria from the human mouth) in 1676. The understanding of bacteria was greatly increased by the work of Louis Pasteur (1822 - 1895), but it was Robert Koch (1843 - 1910) who first linked specific microorganisms to particular diseases, such as tuberculosis, cholera and anthrax. A Viennese surgeon Theodor Billroth (1829 - 1894) coined the term Streptococcus to describe a group of bacteria within the order Lactobacillales and phylum Firmicutes. Beta-hemolytic Streptococci are so-called because when they are cultured on blood agar, the red blood cells are lysed and the hemoglobin the cells contained is denatured so that its red color disappears. Group B beta-hemolytic Streptococci (GBS) are called S. agalactiae (Latin for "without milk") because they cause mastitis in cows. GBS is a facultative gram-positive organism. It is a common bowel commensal in many animals including fish, cattle, and humans, in which it is present in 20 – 40% of adults (2). It can be divided into 10 serotypes based on a serological reaction directed against the polysaccharide capsule, and which are thought to influence virulence and antibiotic resistance. The most frequently identified serotypes that cause invasive disease in neonates are III (60.6%) and Ia (17.3%), whereas type VI (32.7%), Ib (19.4%), and V (19.4%) are the most common cause of invasive disease in adults (3). Serotype VI is the leading type that colonizes pregnant women (35.0%) (3). However, the differences are not currently thought to be sufficient to make them useful in clinical practice.  Figure 1. Group B Streptococcus (GBS) or Streptococcus agalactiae. IntroductionThe primary risk factor for neonatal GBS early-onset-disease is maternal colonization of the genito-urinary and gastro-intestinal tracts. Vertical transmission usually occurs during labor or after rupture of membranes. Implementation of national guidelines for intrapartum antibiotic prophylaxis has resulted in a reduction in incidence of GBS early-onset-disease of more than 80%, from 1.8 newborns per 1,000 live births in the 1990s to 0.23 newborns per 1,000 live births in 2015 (4). In 2010, the Centers for Disease Control and Prevention (CDC), in collaboration with several professional groups, including the American College of Obstetricians and Gynecologists (ACOG), issued its third set of GBS prevention guidelines. In 2018, the stewardship of the change for updating the GBS prophylaxis guidelines were transferred from the CDC to ACOG and the American Academy of Pediatrics (AAP). In addition, the American Society of Microbiology maintains standards for laboratory procedures relevant to processing specimens. The key obstetric measures necessary for effective prevention of GBS early-onset-disease continue to include universal prenatal screening by vaginal-rectal culture, correct specimen collection and processing, appropriate implementation of intrapartum antibiotic prophylaxis, and coordination with pediatric care providers. BackgroundIn 1970s, GBS emerged as an important cause of perinatal morbidity and mortality in newborns (5). Two distinct clinical syndromes of invasive GBS disease in the newborn exist:
PrevalenceApproximately 50% of women who are colonized with GBS will transmit the bacteria to their newborns. In absence of intrapartum antibiotic prophylaxis, 1 – 2% of these newborns will develop GBS early onset disease (8). Among all cases of GBS early-onset-disease, 72% occur in term newborns (9). However, rates of mortality and morbidity related to GBS early-onset-disease are markedly higher among preterm newborns (mortality 19.2% vs. 2.1% respectively). Preterm neonates with GBS early-onset-disease are more likely to experience apnea, require blood pressure support, and need neonatal intensive care (10). Risk Factors Associated with GBS Early-Onset-DiseaseThe primary risk factor for neonatal GBS early-onset-disease is maternal vaginal-rectal colonization with GBS during the intrapartum period. Other risk factors include gestational age less than 37 weeks, very low birth weight, prolonged rupture of membranes, intraamniotic infection, young maternal age, and maternal black race (11). Heavy vaginal-rectal colonization, GBS bacteriuria, and having a previous newborn affected by GBS early-onset-disease also are associated with an increased risk (12). During any trimester, GBS isolated in clean catch urine specimens at any colony count is considered a surrogate for heavy-rectal colonization. Universal Antepartum ScreeningThe CDC first recommended universal antepartum culture-based screening of all pregnant women in the 2002 perinatal GBS guidelines, and universal antepartum culture-based screening continues to be the current standard (13). Regardless of planned mode of birth, all pregnant women should undergo antepartum screening for GBS at 36 0/7 – 37 6/7 weeks of gestation, unless intrapartum antibiotic prophylaxis for GBS is indicated because of GBS bacteriuria during the pregnancy or because of a history of a previous GBS-infected newborn. The 2010 version of the CDC’s perinatal GBS guidelines recommended that prenatal GBS screening be performed starting at 35 0/7 weeks of gestation. The ACOG now recommends performing universal GBS screening between 36 0/7 and 37 6/7 weeks of gestation. The rationale for changing the timing of universal GBS screening is based on two factors:
This change is also likely to reduce the reported incidence of discrepant antepartum culture results and colonization status at the time of birth. In clinical situations in which a pregnant women at term does not give birth within this 5-week screening accuracy window, and whose original GBS screening culture was negative, repeat GBS screening is reasonable and may help guide management beyond 41 0/7 weeks of gestation. Collection and Culture of SpecimenTo maximize the likelihood of GBS recovery, a single swab is used to obtain the culture specimen first from the lower vagina (near the introitus) and then from the rectum (through the anal sphincter) without use of a speculum. Place the swab(s) into a nonnutritive transport medium (e.g., Stuart or Amines medium with or without charcoal). GBS isolates can remain viable in transport media for several days at room temperature; however, the recovery of isolates declines within 1-4 days, especially at elevated temperatures, which can lead to false-negative test results (15).
A key step in this process is incubation of the specimen in enrichment broth before inoculation onto agar culture plates. This method has been shown to maximize GBS identification in culture (16). Molecular-Based (Nucleic Acid) Testing for GBSCurrently, culture-based testing remains the standard for maternal antepartum GBS screening. The laboratory may also use direct latex agglutination tests of nucleic acid amplification testing (NAAT) on the enriched selective broth as an additional or alternative method for processing of antepartum cultures. Rates for GBS detection using NAAT methods have been shown to be equivalent to culture-based screening or better when the test protocol includes and 18 – 24-hour incubation step in enrichment broth before performing the NAAT analysis, which is similar to the process for traditional culture-based methods. Therefore, NAAT-based testing offers a reasonable and potentially more sensitive alternative to a culture for antepartum screening and some laboratories, albeit a minority, report the use of these newer tests for routine antepartum screening (17). NAAT methods for GBS detection also can be used for intrapartum management as a rapid test performed at the time of presentation in labor or for women at term who have unknown or unavailable antepartum GBS screening test results. However, although a 1-2-hour turnaround time is reported when NAAT is uses as a point-of-care test, this time advantage does not allow for the full enrichment broth incubation step that is needed to maximize results. At present, an approach consisting of NAAT-based intrapartum testing alone has not been shown to adequately replace routine prenatal screening at 36 0/7 – 37 6/7 weeks of gestation (18). Indication for Intrapartum Antibiotic ProphylaxisException to universal prenatal GBS vaginal-rectal culture are women who have GBS bacteriuria identified at any time during the current pregnancy and those who have previously given birth to a neonate with GBS early-onset-disease because these risk factors are overriding indications for intrapartum antibiotic prophylaxis (19). See table 1 below 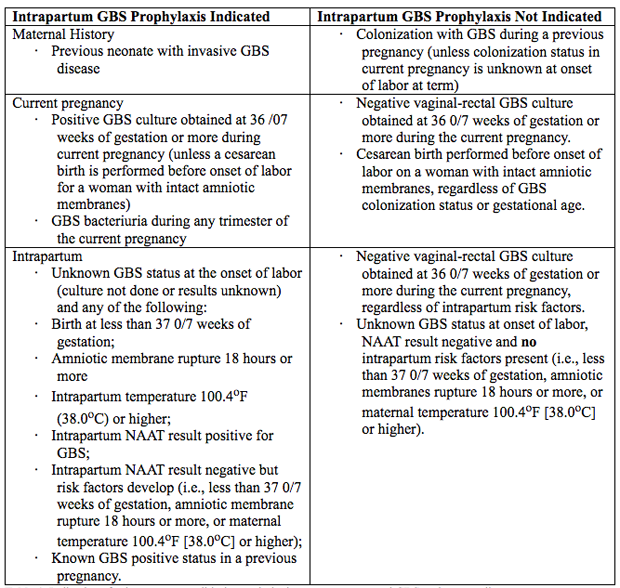 Table 1. Indications for intrapartum antibiotic prophylaxis to prevent neonatal GBS early-onset-disease. BacteriuriaIntrapartum antibiotic prophylaxis indications are:
Preterm LaborAn algorithm for management of women with preterm labor is outlined in Figure 2. Intrapartum prophylaxis for GBS should be started while initial management of possible preterm labor is being undertaken. If preterm labor progresses, intrapartum, antibiotic prophylaxis for GBS should be continued during labor.
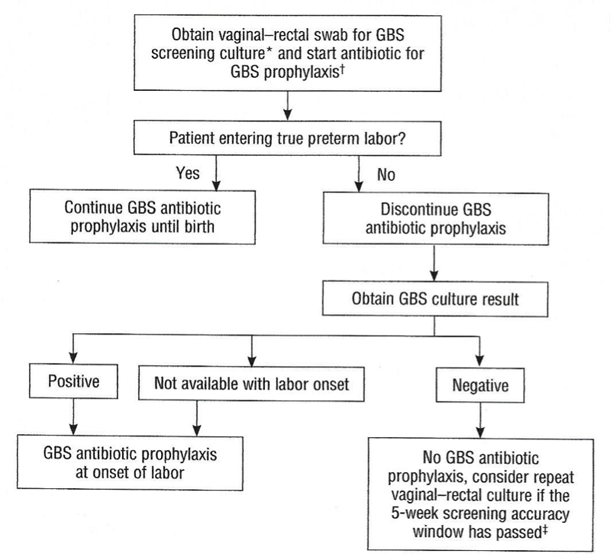 Figure 2. Management of women with preterm labor <37 0/7 weeks of gestation.
Preterm Prelabor Rupture of Membranes (PPROM)An algorithm for the management of women with PPROM is outlined in Figure 3. Current ACOG guidelines recommend proceeding to delivery if PPROM occurs at or beyond 34 0/7 weeks of gestation (23). 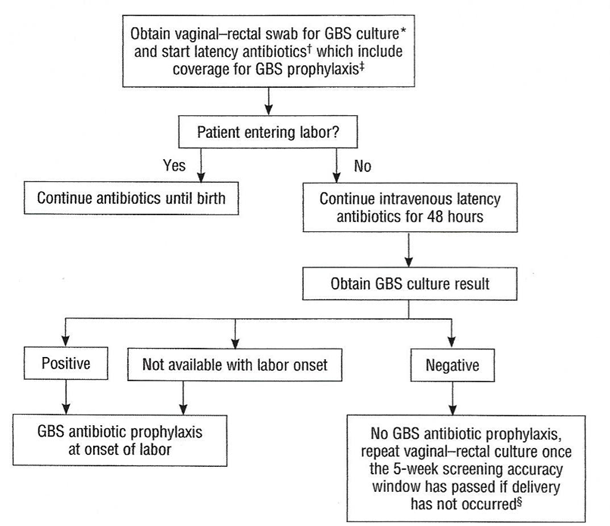 Figure 3. Management of women with Preterm Prelabor Rupture of Membranes (PPROM). If a patient with PPROM is entering labor and had a negative GBS culture more than 5 weeks previously, she should be rescreened and managed according to this algorithm (see figure 3) at that time. Two secondary analyses of large multicenter randomized controlled trials of PPROM in women colonized with GBS found a lower risk of neonatal infect associated with immediate induction in women who were later preterm (34 0/7 – 36 6/7 weeks of gestation) and early term (37 0/7 – 38 6/7 weeks of gestation) (24). In such cases, immediate induction rather than extended expectant management is recommended. Planned Cesarean BirthIntrapartum prophylaxis that is specific for GBS is not recommended for women undergoing a planned cesarean birth in the absence of labor and PPROM, regardless of the gestation age, even among women who are GBS positive. Multistate surveillance reveals that GBS early-onset-disease occurs at a very low rate in this situation, approximately 3 per 1,000,000 live births (24). This does not change the recommendation that women undergo cesarean birth (regardless of GBS colonization status) be administered one dose of prophylactic antibiotics before the incision to reduce the risk of postoperative infections (22). Women planning cesarean birth should nonetheless undergo prenatal GBS culture at 36 0/7 - 36 6/7 weeks of gestation because onset of labor or PPROM may occur before the planned cesarean birth. Should a woman with a planned cesarean birth and a positive antepartum GBS culture present in active labor or with PPROM before her scheduled delivery date, a single dose of an antibiotic (or combination of antibiotics) that provides GBS prophylaxis and presurgical prophylaxis is appropriate. In most clinical situations, cefazolin will meet both these criteria. Delaying the cesarean birth to administer additional doses of antibiotics for GBS prophylaxis alone is not indicated. Unknown Culture Status During Labor at TermThere are three ways to identify candidates for intrapartum antibiotic prophylaxis when a woman at term presents in labor with unknown GBS culture status and does not have an established indication for intrapartum antibiotic prophylaxis. In this situation:
When a woman is in labor and her GBS colonization status is unknown, a temperature of 100.4oF (38oC) or higher, or rupture of membranes for 18 hours or more, is independently associated with an increased risk of neonatal GBS early-onset-disease (25). Although reduction of neonatal prophylaxis, if suspected or confirmed intraamniotic infection develops, intrapartum antibiotic prophylaxis targeted against GBS should be converted to a more broad-spectrum antibiotic regimen for treatment of intraamniotic infection that includes activity against GBS, generally ampicillin and an aminoglycoside (26). Intrapartum Antibiotic ProphylaxisIntrapartum antibiotic prophylaxis to reduce the risk of GBS early-onset-disease is based on a two-pronged approach:
Antimicrobial AgentsIntravenous penicillin remains the agent of choice for intrapartum prophylaxis, with intravenous (IV) ampicillin as an acceptable alternative. Penicillin is the preferred first-line agent because it has a narrow, more targeted spectrum of antimicrobial activity against gram-positive bacteria and lower likelihood of inducing resistance in other vaginal organisms. The current recommendations are (27): 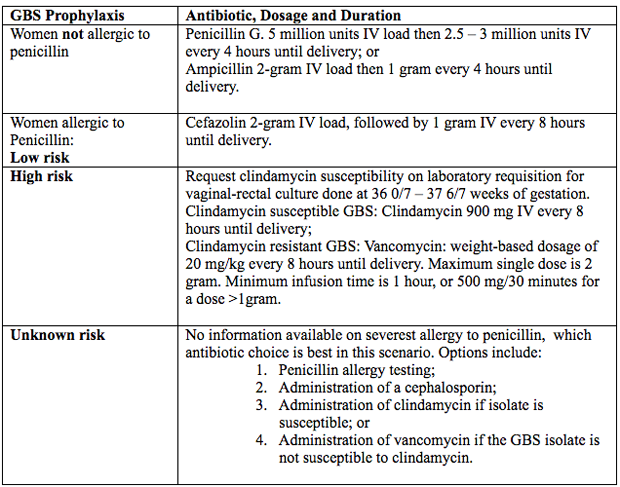 Figure 4. Intrapartum antibiotic prophylaxis. Abbreviations: GBS (group B streptococcus); IV (intravenous) Penicillin Allergy in PregnancyPenicillin was first used almost 80 years ago in 1941, to successfully treat a person with a life-threatening streptococcal infection. Over the remainder of the 20th century, advances in antibiotic therapy led to a decrease in infections as the cause of mortality in the United States from, 33% to 4.5% in 1999 (28). In obstetrics and gynecology, these classes of antibiotics are commonly used to treat urinary tract, intrapartum, postpartum and sexually transmitted infections. One of the most common uses of these antimicrobial agents is intrapartum prophylaxis for GBS colonization. Penicillin allergies has led to routine use of alternative but potentially less effective agents. Individuals treated with alternative antibiotic regimens in the setting of a presumed penicillin allergy have also been shown to have longer-duration hospitalizations (29). Furthermore, broad-spectrum antibiotics are often more costly. A specific and common example of this problem in obstetrics is the need to incorporate alternative penicillin-antibiotic regimens for intrapartum antibiotic prophylaxis against neonatal GBS early-onset-disease owning to a reported, frequently undocumented, maternal history of penicillin allergy. Importantly, most individuals who report a penicillin allergy are neither truly allergic nor at risk of a developing a hypersensitivity reaction after exposure to penicillin. Approximately 80 – 90% of persons with a history penicillin allergy are actually penicillin tolerant (29). There are a number of reasons for this seeming contradiction. First, the risk of recurrence for even IgE-mediated penicillin reaction has been shown to dissipate over time. It has been reported that elicitable sensitivity to penicillin is no longer present by 5 years after a reported IgE-mediated reaction in approximately 50% of individuals, and this rate increases to at least 80% by 10 years after the reaction (30). Thus, many adults today have a history of penicillin allergy that does not represent a risk for true recurring penicillin hypersensitivity that would preclude its subsequent use. In addition, a common confounding scenario that can result in a presumed penicillin allergy occurs when the original adverse reaction was not directly related to penicillin. Evaluation of Penicillin AllergyQuestions and interpretation of answers below, can help determine the probability that the allergic reaction was an IgE-mediated hypersensitivity reaction or rare severe form of T-cell response (31).
Definitions of Penicillin AllergyThe determination of risk is further confounded by a lack of standard classifications or terminology among national medical societies and individual experts to facilitate distinguishing allergy risk categories. In addition, there are no universally recommended allergy history questionnaires to assist with clinical assessment, though several have been evaluated in published research in attempts to validate their effectiveness as components of penicillin allergy evaluation guidelines. The following definitions are recommended by ACOG (19). Low Risk: High Risk: Penicillin Allergy TestingFormal targeted drug allergy testing assessments can confer real benefit to patients, either to substantiate the reported adverse drug reaction, especially when the historical recollection of allergy is unclear, or to exclude it. Penicillin allergy testing has been shown to be safe to perform and can be beneficial for all women who report a penicillin allergy, particularly those whose symptom descriptions are suggestive of being IgE-mediated, of unknown severity, or both. Pregnancy presents a unique challenge owing to both maternal and provider concerns that the allergy evaluation itself may have an untoward effect not only on the woman but on her fetus as well. Two small studies have described the safety and performance of penicillin skin testing in pregnant women who reported a penicillin allergy and were subsequently screened as positive GBS colonization in the 3rd trimester. Between 93% to 96% of these women underwent successful outpatient penicillin allergy skin testing without any complications and subsequently were able to receive penicillin as intrapartum antibiotic prophylaxis for their GBS colonization (32). More recent consensus guidelines advocate for pregnant women with allergy histories and negative penicillin skin allergy testing to have follow-up confirmation with an oral amoxicillin challenge (33). In locations without access to skin-testing materials, an oral amoxicillin challenge test only (without skin-testing initially) for low-risk patients has been recommended (33). The logistics of a protocol for testing in pregnancy include: a requirement that the clinical sites be staffed by the necessary personnel (such as physicians, pharmacists, and nurses trained in penicillin allergy testing), that required materials for the penicillin skin testing be available on site, and that management protocols be in place, with appropriate medications readily available, in the uncommon event of a drug challenge reaction. This may require the testing to be performed in a setting that can accommodate a short length of stay (several hours) and continued monitoring of both the woman and her fetus during the testing process, depending on gestational age. Maternal Obesity and Cord Blood Penicillin LevelsIt has been shown in other clinical scenarios that higher antibiotic doses are required to achieve adequate therapeutic effects in patients with obesity. Both pregnancy and obesity can alter the pharmaco-kinetics and pharmaco-dynamics of many medications, and β-lactam antibiotics have been shown to have and increased volume of distribution in patients with obesity (34). This increased volume of distribution can lead to diminished therapeutic benefit at the relevant tissue. Maternal obesity is a known risk factor for neonatal GBS disease, though this risk has generally been attributed to higher rates of GBS colonization among women with obesity. However, these data raise the question of whether the recommended intrapartum antibiotic prophylaxis dose could possibly be inadequate in women with obesity. There are limited data about the pharmaco-kinetics of penicillin in women in the third trimester of pregnancy, especially in the setting of obesity. No data exist to confirm that the standard dosing of penicillin for intrapartum prophylaxis achieves adequate therapeutic concentration in patients with obesity. This prospective cohort study (35), of term women receiving intrapartum penicillin prophylaxis for GBS colonization (determined by antenatal rectovaginal culture). The following outcomes were compared between obese (body mass index [BMI] 35 or higher at delivery) and non-obese (BMI less than 30 at delivery) groups: penicillin concentration in maternal blood (after two penicillin doses) and umbilical cord blood, GBS rectovaginal colonization status on admission and after two completed doses, and neonatal GBS colonization (using a postnatal ear swab). 55 women were needed to detect a 0.75 SD difference in cord blood penicillin concentration. Conclusion: the median cord blood penicillin concentration was 60% lower in neonates born to women with obesity. However, all concentrations exceeded the minimum inhibitory concentration. Maternal penicillin levels were not significantly different between groups. More than 40% of women who previously tested positive for GBS by antenatal culture tested negative for GBS on admission for delivery (35). Continued research is needed to better understand optimal medication dosing in pregnant women with obesity, and to ensure that the GBS screening and intrapartum antibiotic prophylaxis protocols are as precise and effective as possible. Recommendations and Key Points
BreastfeedingBreastfeeding shapes the neonatal gut microbiota, both because of exposure to the milk microbiota and via factors such as milk oligosaccharides, secretory IgA and antimicrobial antibodies, which are thought to guide the infants’ developing mucosal immune system (36). There has been a concern that some cases of the late-onset GBS disease are secondary to transmission via the mother’s milk, but in other cases the immune factors in breast milk may be protective. One small study has suggested that, results demonstrate the benefit of continued breastfeeding after emergency cesarean section, in promoting a positive-weaning gut microbiota profile comparable to vaginally born infants without intrapartum antibiotic prophylaxis, but no such ameliorating effect was seen with babies born vaginally (36). Any effect of breast feeding on the microbiota following intrapartum needs further study. SummaryUniversal prenatal, culture-based screening for maternal GBS colonization and intrapartum antibiotic prophylaxis together currently constitutes the most effective strategy for reducing perinatal morbidity and mortality secondary to GBS. To date, this regimen has been associated with a significant decrease in the incidence of GBS early-onset-disease and has not been associated with adverse effects in women or newborns. Intrapartum screening using NAAT for GBS has been shown to have high sensitivity and specificity, but many of these tests need several hours of enrichment to attain that level of performance, which limits their value if a result is needed rapidly. Appropriate collection of vaginal-rectal GBS screening cultures, proper use of indicated antibiotics, and optimization of the correct application of intrapartum antibiotic prophylaxis, along with educational efforts to reinforce understanding of those practices, are key to minimizing the risk of GBS early-onset-disease. As many as 30% of pregnant women will receive some type of antibiotic therapy over the course of pregnancy, labor and birth, with penicillin being the most prescribed antibiotic used for antepartum and intrapartum prophylaxis in women who have screened positive during pregnancy for GBS colonization. Still, reported penicillin allergies prevent women from receiving this first-line-narrow-spectrum antibiotic when indicated, while they may have implications for prevention of neonatal infection. antibiotic stewardship initiatives designed to focus, limit and guide the use of these potent but fragile pharmacologic agents in practice are a critical component of optimizing care across all health care facilities. The long-term benefits of evaluating penicillin allergy, especially prenatally, include the ability to use the first-line antimicrobial agents proven to be most effective when needed, as well as the possibility of lowering the rates of complications related to both infections and their treatments. We advocate for wider consideration and adoption of penicillin allergy testing in pregnant women specifically and the female population in general. Suggested ReadingGroup B Streptococcal Perinatal Infection: A Comprehensive Review |