Understanding Assisted Reproductive TechnologyDr. Bradley J. Van Voorhis
Professor and Director of Reproductive Endocrinology
University of Iowa, Iowa City, IA (USA)Over the past two decades, the use of assisted reproductive technology (ART) has increased dramatically worldwide and has made pregnancy possible for many infertile couples. ART encompasses all techniques involving direct manipulation of oocytes outside of the body. Because of intense research efforts, pregnancy rates with ART have shown continuous improvement. However, in recent years, outcomes other than live birth rates have become an important focus of investigation. Problems including multiple gestations, ovarian hyperstimulation, and the health of children born from these techniques have gained new prominence as researchers are now evaluating the health consequences of these procedures. The success of modern ART has completely revolutionized both the evaluation and treatment of infertility. The purpose of this document is to provide an understanding, overview and indications for assisted reproductive technologies (ART). The results and complications of ART with an emphasis on newly developing technologies and areas of controversy are also discussed. Pregnancy rates after ART have shown nearly continuous improvement in the years since its conception. A number of factors affect the pregnancy rate, with the most important being a woman's age. Many studies are now finding that there is a slight increase in adverse pregnancy outcomes after ART. Although the vast majority of children born from these procedures are healthy, there is some concern about increased rates of prematurity, small for gestational age infants and a slight increase in the rates of birth defects following ART. Some of these complications can be linked to the problem of multiple gestations which are common following ART. Definitions and Terminology:The American Society for Reproductive Medicine defines ART as treatments and procedures involving the handling of human oocytes and sperm, or embryos, with the intent of establishing a pregnancy (1). It includes all fertility treatments in which both eggs and sperm are handled in vitro. It excludes techniques such as artificial insemination and superovulation drug therapy. The large majority of ART cycles are in vitro fertilization (IVF) cycles, performed by culturing embryos for variable numbers of days before transferring the embryos through the cervix to the uterus. ART technology includes the use of donor eggs and cryopreserved embryos. In most cases, IVF is used to help an infertile couple conceive and carry their own biological child, but donor sperm, donor oocytes, and gestational surrogates can also be used, alone or in combinations, as circumstances require. The first and still most common procedure is in vitro fertilization (IVF), but there are other procedures used in conjunction with or in place of IVF: IVF -- In Vitro Fertilization: extraction of oocytes, fertilization in the laboratory, transcervical transfer of embryos into the uterus.
GIFT -- Gamete Intrafallopian Transfer: the placement of oocytes and sperm into the fallopian tube.
ZIFT -- Zygote Intrafallopian Transfer: the placement of fertilized oocytes into the fallopian tube.
TET -- Tubal Embryo Transfer: the placement of cleaving embryos into the fallopian tube.
ICSI -- Intracytoplasmic sperm injection (of a single spermatozoon into an oocyte to facilitate fertilization). ART now also includes methods for assisted fertilization by intracytoplasmic sperm injection (ICSI) using sperm isolation from the ejaculate or obtained by microsurgical epididymal sperm aspiration (MESA) or testicular sperm extraction (TESE), assisted embryo hatching, and preimplantation genetic diagnosis (PGD). In past years GIFT, ZIFT, TET, these more invasive techniques offered certain advantages over traditional IVF for some infertile couples, but no longer. Consequently, they now have only limited indications. Indications for IVF:The birth of the first child from in vitro fertilization (IVF) occurred just over 25 years ago. IVF was first developed as a means to overcome infertility resulting for irreparable tubal disease, but it is now applied much more broadly for the treatment of almost all causes of infertility. Its use has grown to the point that nearly 1% of babies born in the United States are now conceived by IVF. In women with premature ovarian failure and healthy women beyond the normal range of reproductive age, IVF using oocytes from a young donor is highly successful. For women with normal ovaries but no functional uterus (Mullerian agenesis, severe intrauterine adhesions, previous hysterectomy) and those with medical disorders in whom pregnancy would pose a serious health risk, IVF with embryo transfer to a gestational surrogate still offers the possibility of genetic offspring. In couples who carry autosomal-recessive or sex-linked genetic disorders or a balanced chromosomal translocation, IVF with preimplantation genetic diagnosis (PGD) can be used to avoid the risk of delivering an affected child. - Tubal Factor Infertility -- steady advances in ART have improved IVF outcomes to where they now far exceed what can be achieved with tubal reconstructive surgery. Surgical treatments for tubal factor infertility are generally in an era of decline; laparoscopic surgery has replaced simple open procedures, and ART has replaced more complicated ones. Tubal surgery remains a legitimate treatment option for women seeking pregnancy after a previous tubal sterilization, for those with mild distal tubal disease (particularly when they are young), and for women with apparent proximal tubal occlusion. Under virtually all other circumstances, IVF is the best and most logical choice. Salpingectomy should also be viewed as an effective adjunct to IVF for women with large hydrosalpinges.
- Endometriosis -- the weight of available evidence indicates that both mild and severe endometriosis have adverse effects on fertility. In women with advanced endometriosis, abnormal reproductive anatomy has important impact, but regardless of the stage of disease, other mechanisms involving intrinsic or disease-induced abnormalities of oocyte development, embryogenesis, and endometrial function likely contribute to infertility. After surgical treatment, the choice between expectant or empirical treatment and IVF should be based on age, the surgical results, and the severity of any other coexisting infertility factors.
- Male Factor Infertility -- when treatment is not possible or fails and insemination with donor sperm is not an acceptable option, IVF and ICSI, using sperm isolated from the ejaculate or extracted from the epididymis or testis, offer a very realistic hope for success. Additional genetic evaluation is indicated for men with severe oligospermia (sperm concentration less than 5 million/mL) whose sperm may be used for ICSI. When intrauterine insemination (IUI) is not possible, or the prognosis for success with IUI is poor, or IUI proves unsuccessful and therapeutic donor insemination is rejected, IVF and ICSI is the logical alternative (2).
- Unexplained Infertility -- among couples with unexplained infertility, IVF is the preferred treatment for some and the treatment of last resort for others. A number of groups have observed a higher incidence of fertilization failure and lower overall pregnancy rates in couples who have already failed treatment with gonadotropins/ IUI, suggesting that abnormalities of fertilization, early embryonic development or implantation might be responsible for unexplained fertility in some couples (3).
- Ovarian Failure / Diminished Ovarian Reserve -- donor oocyte IVF is most commonly performed in women over age 42 and others in whom the results of ovarian reserve testing indicate an extremely poor prognosis for success with IVF using their own oocytes. Women with inaccessible ovaries and those in whom IVF repeatedly yields poor quality embryos are also candidates for donor oocyte IVF.
- Other Indications for IVF -- women recently diagnosed with a cancer or another medical disorder facing imminent treatment (chemotherapy, radiation therapy) that poses a serious threat to their future fertility may be candidates for IVF and cryopreservation of embryos before treatment begins, if time and health allow. Women with normal ovaries but no functional uterus as a result of a congenital anomaly (Mullerian agenesis), advanced disease (multiple myomas; severe intrauterine adhesions), or a previous hysterectomy, and those with medical conditions in which pregnancy poses a serious health risk may still be offered the opportunity to have their own genetic offspring via IVF with transfer of embryos to the uterus of a gestational surrogate. Anovulatory women who ovulate in response to treatment but do not conceive also may ultimately become candidates for IVF. Women who carry a genetic risk or disorder that may be expressed in their offspring may be candidates for IVF with preimplantation genetic diagnosis to identify and exclude affected embryos. There is growing interest in the application of preimplantation genetic diagnosis for "aneuploidy screening" among the embryos of older women, those with a history of recurrent early pregnancy loss, and women with repeated unexplained IVF failures despite transfers of morphologically normal embryos.
Prognostic Factors for Pregnancy After ART:Younger women and those with a normal ovarian reserve are more likely to achieve pregnancy than older women and others with a diminished ovarian reserve. Women with a previous live birth are more likely to succeed than nulliparous women; success rates are also lower for women with a previous failed IVF cycle. However, a history of an earlier unsuccessful pregnancy (miscarriage) has no effect on the likelihood for success with IVF. All smoking women should be strongly encouraged to stop smoking before IVF because smoking decreases the likelihood for success by up to one-half.- Demographic Factors -- a woman's age is the most important factor affecting the chances of live birth after ART. From 2002 CDC data, live birth rates per cycle range from just over 40% in women aged 27 years, down to 6% at age 43, and only 2% in women who are over 43 years of age. A large increase in miscarriage rate with aging (reaching nearly 45% at age 43) contributes to the low live birth rate after IVF in relatively older women. Data from donor oocyte cycles demonstrate that the reduced fertility associated with aging is linked primarily to aging of the ovary and oocyte rather than aging of the uterus and endometrium. Women who have been pregnant, but miscarried, have the same pregnancy rate as age-matched women who have never been pregnant. Among women aged 40 or younger, those who have had no previous IVF cycles have a slightly higher pregnancy rate than those women who have had 1 or more previous IVF cycles that have not resulted in pregnancy.
- Infertility Diagnosis -- compared with the average live birth rate of 28.3%, a distinctly lower live birth rate of 13.9% is seen with the diagnosis of reduced ovarian reserve. Women with multiple diagnoses had a live birth rate of 23.4%, and couples with both male and female diagnoses had a live birth rate of 26.4%, as per 2002 CDC data. Uterine factor infertility, defined as a structural or functional disorder of the uterus, was associated with a reduced pregnancy rate of 22.9%. In contrast, very little difference in pregnancy rates is seen when comparing couples with the diagnosis of tubal factor infertility, or unexplained infertility. Couples in all of these diagnostic groups had similar live birth rates per cycle of 30-50%. The effect of endometriosis on ART outcomes is difficult to determine from national data due to selection basis, because not all cases have been ascertained in the diagnostic evaluation of infertile couples.
- Hydrosalpinges and Pregnancy after ART -- the mechanism of the impaired pregnancy rates with hydrosalpinges (dilated fallopian tubes due to obstruction often following tubal infection) and IVF is unknown. Theories have focused on the toxic effect of this fluid on embryo development, the effect of the fluid on endometrial receptivity and implantation, and the simple mechanical wash out of embryos by hydrosalpinx fluid (4). Each of these theories is supported by in vitro data, but the true cause of reduced pregnancy rates is unknown. Retrospective data suggest that women who had removed by salpingectomy have an improved pregnancy rate after ART treatment. In our clinical practice, we perform ultrasound examinations of all women who are suspected of having tubal disease, either by clinical factors or based on hysterosalpingogram. If a hydrosalpinx is present, we always determine whether or not ART is an option for the couple before performing a laparoscopic procedure. If ART is possible, it has been our practice to perform laparoscopic salpingectomy for its beneficial effects on subsequent ART cycles.
- Leiomyomata and ART Outcomes -- the effect of uterine leiomyomata on outcomes with ART likely depends on the size and location of the tumor. Current expert opinion is that uterine leiomyomata that are intracavitary or distort the endometrium have an adverse effect on live birth rates after ART. Patient symptomatology and reproductive history is necessary in deciding whether or not to perform surgery for intramural leiomyomata not impinging on the endometrium.
- Smoking and ART Outcomes -- it reduces the pregnancy rate by approximately by 50%. The mechanism of this effect is not clear, but constitutes cigarette smoke can be detected in follicular fluid and thus could affect the health of oocytes or embryos.
- Decreased Ovarian Reserve -- it is defined by poor ovarian follicular response to gonadotropin stimulation during ART, probably secondary to a reduced number and quality of ovarian follicles available for stimulation. This results in a much higher rate of cycle cancellation, fewer eggs retrieved, fewer embryos, and a lower pregnancy rate with ART. The most widely used test for estimating ovarian reserve is a basal FSH value (5). The basal FSH is typically drawn on cycle day 2, 3, or 4, and values above 15 mIU/mL (in many laboratories) suggest a decreased ovarian reserve and a significantly reduced probability of pregnancy after ART. If the FSH is above 20 mIU/mL, the chances for pregnancy are virtually zero. Another test for ovarian reserve is the clomiphene citrate challenge test. This test requires a basal FSH value on cycle day 3, followed by the administration of 100 mg of clomiphene citrate on cycle days 5-9. A second FSH value is then obtained on cycle day 10. If either FSH value is elevated, reduced ovarian reserve is diagnosed. It has been suggested that ovarian reserve testing reflects oocyte numbers but not quality, and thus older women (with reduced egg quality) still have a low chance of pregnancy with ART even after the finding of a normal FSH value. Reduced ovarian reserve can also be diagnosed through ultrasound observation of the ovaries. Many investigators have found that low numbers of ovarian antral follicles (<10 total follicles with a diameter between 2 and 10 mm) indicates reduced ovarian reserve and diminished chance for pregnancy after ART.
- Laboratory Techniques -- one innovation is prolonged culture of embryos before transfer to the uterus. More prolonged culture (often for 3-5 days) allows embryologists to observe embryos for growth and morphology and select presumably "healthier" embryos for transfer to the uterus. The disadvantage of prolonged culture is that average rates of blastocyst formation have ranged from 28% to nearly 50% in various series. Thus, more embryos need to be cultured to produce a suitable number of blastocyts for transfer, and some women may not have a good-quality blastocyst to transfer. Blastocysts appear to have a higher implantation rate than embryos transferred at cleavage stages (day 2 or day 3), allowing for transfer of fewer embryos to achieve the same pregnancy rate. Although the pregnancy rate may not differ, the major advantage of prolonged culture may ultimately be a reduction in multiple gestations after ART (6).
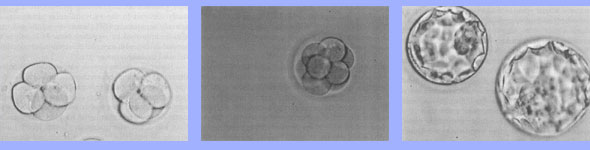 | | Human embryos cultured in vitro. 1) Embryos after 2 days of culture at the 4 cell stage of development. 2) An 8 cell embryo on culture day 3. 3) Blastocyst embryos on culture day 5. All photographs were taken at the same magnification. |
Adverse Outcomes from ART: Multiple Gestations -- with ART, twining rates are 22 times higher than what is seen in the general population, and triplets and higher order multiples are 50 times the natural rate of 0.18% (7). Nationally, it has been estimated that more than 40% of the triplet and higher-order births in 1997 were the result of ART and another 40% due to use of ovarian-induction drugs. Although multifetal births account for only 3% of all live births nationally, they account for 17% of all preterm births (<37 weeks of gestation), 23% of early preterm births (<32 weeks of gestation), and 26% of very low birth weight infants (<1,500 g). Children born after IVF have been found to have an increased risk of cerebral palsy and a higher hospitalization rate, mainly due to the high twining rate associated with IVF. Parents of twins experience more marital stress than did parents of singletons. Multiple gestations also increase the maternal risks of hypertension, postpartum bleeding, premature labor with prolonged bed-rest, and cesarean deliveries. Although rare, maternal mortality is increased in multiple gestations. Perinatal Outcomes for Singletons Conceived after ART -- the accumulated data suggest that even singleton pregnancies conceived after IVF are at higher risk for adverse outcomes. It has been noted a 2-fold increase risk for perinatal mortality, low birth weight, very low birth weight, preterm delivery, and small for gestational age infants (8). Increased risks for gestational diabetes, placenta previa, preeclampsia, and still birth are also found among IVF-conceived singletons. The odds ratios for these complications ranged from 1.55 for preeclampsia to 2.87 for placenta previa. The cause of these adverse perinatal outcomes among IVF-conceived singletons is unknown. One possibility is that some aspect of IVF treatment (eg, ovarian stimulation or embryo culture) may increase the risk of subsequent adverse pregnancy outcomes. Alternatively, there may be an underlying disorder in the infertile couple that contributes both to the infertility and the adverse perinatal outcomes. Birth Defects after ART -- Whether or not birth defects are increased after ART is controversial. Several studies have shown an increased risk although the absolute increase has been low in all studies. For example, one recent study found the incidence for a major birth defect in IVF-conceived children is 6.2% compared with a rate of 4.4% in naturally conceived children. Recent attention has been directed toward epigenetic errors that might be inherent in the infertile couple or induced as an adverse effect of ART itself. Prolonged exposure to embryo culture media used in IVF may predispose to imprinting defects in the human embryo as has been demonstrated in bovine embryos cultured in vitro under certain conditions. It is important to realize that the number of infants affected by known imprinting disorders after ART is extremely small. Birth Defects after ICSI -- male children conceived after ICSI carry the same Y chromosome microdeletions as their fathers. In addition, a higher rate of karyotypic abnormalities has been described in pregnancies conceived after ICSI than in pregnancies conceived by IVF with standard insemination. Furthermore, the incidence of karyotypic abnormalities after ICSI can be correlated with the number of sperm in the ejaculate, showing the link between the severity of male factor infertility and subsequent chromosomal aberrations in offspring. Of course, the children born from ICSI are too young to yet know the effect of these genetic abnormalities on their subsequent reproduction. Ovarian Hyperstimulation Syndrome -- it can occur in mild form, consisting or increased ovarian size accompanied by abdominal discomfort. Severe ovarian hyperstimulation syndrome is characterized by significant abdominal distention and pain, often accompanied by nausea and vomiting. The abdominal distension is secondary to enlarged ovaries and a protein-rich ascites accumulating in the peritoneal cavity but also occasionally in the pleural and paracardiac space. Ovarian hyperstimulation syndrome is a self-limiting process that gradually resolves over several weeks although patients may need to be supported during this time by intravenous fluid hydration, pain control, and paracenteses to control ascites and reduce distension of breath. Cost-Effectiveness of ART:There is no question that IVF is expensive. The initial estimates of the cost/delivery of IVF ranges somewhere between $44,000 and $212,000 per delivery but more recent studies have reported lower estimates reflecting higher pregnancy rates with IVF over time. In certain cases of male factor infertility, ART using ICSI may be more cost-effective than attempts at conception using IUI. From a cost-effectiveness standpoint, ART may be the favored initial treatment for couples with certain causes of infertility. For women with tubal disease, all published studies agreed that IVF is at least as cost-effective as tubal surgery. There is no question that outcomes from tubal surgery will depend greatly on the degree of tubal damage. However, even experienced surgeons have reported live birth rates of only 20-30% after surgery for distal tubal occlusion, and these rates are generally achieved only after 2-3 years of follow-up compared with higher pregnancy rates achieved in a single month of IVF treatment.Summary:Assisted reproductive technology (ART) is one of the great success stories in the field of obstetrics and gynecology. Pregnancy rates continue to improve because of new laboratory techniques and recognition of clinical factors that impact outcomes. Although generally safe, adverse outcomes in the short term have been described both in the women undergoing ART and in infants born from these procedures. Continued research is required to understand the causes of these adverse consequences. Of great importance is the need to make ART safer by reducing the incidence of multiple gestations. Editor's note: Once again, we at Women's Health and Education Center (WHEC) would like to acknowledge the contributions of Dr. Bradley J. Van Voorhis in preparing this manuscript. His expert opinions, technical skills and friendship helped us in the development of this chapter. References:- ACOG Committee Opinion. Perinatal Risks Associated with Assisted Reproductive Technology. Number 324, November 2005.
- Lee RK, Hou JW, Ho HY et al. Sperm morphology analysis using strict criteria as a prognostic factor in intrauterine insemination. Int J Androl. 2002;25:277-281
- Martin JS, Nisher JA, Parker JI et al. The pregnancy rates of cohorts of idiopathic infertility couples gives insights into the underlying mechanism of infertility. Fertil Steril. 1995;64:98-102
- Strandell A. The influence of hydrosalpinx on IVF and embryo transfer: a review. Hum Reprod Update. 2000;6:387-395
- Bukman A, Heineman MJ. Ovarian reserve testing and the use of prognostic models in patients with subfertility. Hum Reprod Update. 2001;7:581-589
- Van Voorhis BJ. Outcomes from Assisted Reproductive Technology. Obstet Gynecol. 2006;107:183-200
- Lynch A, McDuffie R, Murphy J et al. Assisted reproductive interventions and multiple births. Obstet Gynecol. 2001;97:195-200
- Schieve LA, Ferre C, Peterson HB et al. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103:1144-1153
©
Women's Health and Education Center (WHEC)
|
