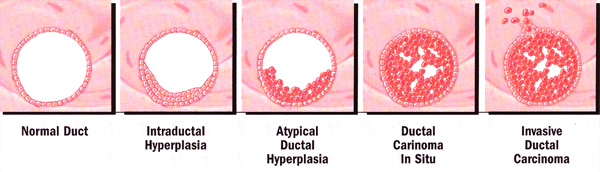
Breast Cancer: Early DetectionIt is estimated that 217,640 new cases of breast cancer will be diagnosed in United States this year; more than 99% of these breast cancers occur in women. Breast cancer is the most common malignancy among U.S. women and it is the second leading cause of death from cancer among American women (lung cancer is first). A woman's lifetime risk (80-year life-span) of developing breast cancer is 12.5%, or 1 in 8. Early detection of breast cancer has been shown to decrease the mortality rate. Technology continues to evolve to improve the accuracy of detection. The purpose of this document is to discuss the rationale for current breast cancer screening guidelines and evaluate the evidence regarding screening techniques. It also focuses on mammography and other detection techniques as screening tools to identify non-palpable lesions. This information is designed to aid practitioners in making decisions about appropriate care. Variations in practice may be warranted based on the needs of the individual patient, resources, and limitations unique to the institutions or type of practices. The analytic epidemiology - why women acquire breast cancer is less well understood. Several factors have been identified with increased risk, such as: benign breast disease, genetic factors, diet, exogenous hormones, alcohol and tobacco consumption, breast trauma and viruses. Risk of breast cancer increases by being a woman and growing older; 70% of cancers occur over age 50 years. Family history of breast cancer in a primary relative (mother, sister or daughter) increases the risk. Other factors are: early onset of menses (getting period before age 12 years), never giving birth or first pregnancy over age 30 years, weighing more than 40% above the ideal body weight and exposure to DDT (pesticide), PCB, or excessive radiation to the chest wall. Several studies have demonstrated that the most important pathologic risk factors for the subsequent development of carcinoma are the degree and nature (typical or atypical) of epithelial proliferation. Breast pathology diagnosis grouped by cancer risk (1): Screening tools are only as effective as their ability to aid in disease prevention. The ideal screening method should identify biologically and statistically significant changes associated with cancer development. It should also be applicable to a reasonable proportion of at-risk individuals, and it should be minimally invasive. Additionally, effective intervention and preventive measures should be available. The search for better methods of breast cancer prevention and detection continues with the goal of reducing mortality significantly, as has occurred with cervical cancer. Obstetricians and gynecologists are in a favorable position to diagnose breast disease in their patients. Initial step towards this is the breast examination by visual inspection and palpation and it should be integral part of initial obstetric and all complete gynecologic examinations. Patients should be instructed in the technique of life-long periodic self-examination of the breast and informed of the importance of self-examination. Despite a lack of definitive data for or against breast self-examination (BSE), breast self-examination has the potential to detect palpable breast cancer and should be recommended. Breast cancer is the most self-discovered of all cancers. In fact, most breast cancers in the United States are detected by palpation. Breast self-exams (BSE) should be done in a comfortable room that contains a bed (including a pillow), a mirror and good lighting. You may perform BSE when you are in the shower; some women find that a wet hand is helpful in detecting changes in their breasts. Remember to perform BSE while lying down in addition to standing up. Breasts are dynamic organs and changes can occur monthly and throughout life stages (adolescence, child-bearing, menopause). US Food and Drug Administration has approved BSE aids that are designed to improve tactile sensation. It is a good idea to draw a sketch of what you feel in your BSE and to mark your calendar. Keep in mind that most breast lumps are not cancerous. In fact, it has been estimated that only one in 11 lumps is a tumor. BSE is free and non-toxic and some physicians believe that BSE helps empower women to take control of their own health. Proper monthly breast self-exam, several days after the end of the menstrual cycle and clinical breast exam by a trained healthcare provider once a year, is suggested. The majority of women diagnosed with breast cancer have no obvious risk factors. In 1966, Macdonald estimated invasive cancer doubling times to range from 23 to 209 days, with an average of 128 days for localized cancer and 85 days for metastatic cancer. A graph based on mathematical calculation of 100-day doubling time revealed an 8-year pre-clinical phase and a 4-year clinical phase of breast cancer growth, which occurs before the usual size when breast cancer is diagnosed. Mammography screening could potentially identify a non-palpable mass of approximately 1 mm to 1 cm during its pre-clinical phase 3 years before it becomes palpable (2). Most studies of mammography use mortality as the end-point, so the value of screening often is solely based on mortality rates. Few studies take into account the effects of early detection on quality of life or the fact that treatment at earlier stages carries less morbidity. In 1982, the Breast Cancer Detection Demonstration Project established the applicability of breast cancer screening with mammography to identify non-palpable lesions. The 5-year result demonstrated that 8.7% of breast cancers were detected by physical examination only, 41.6% were detected by mammography only, and 47.3% were detected by both mammography and physical examination. Of infiltrating breast cancer smaller than 1 cm, 52.6% were detected only by mammography. Among the 4,240 women in whom breast cancer was detected and treated, the 10-year survival rate was 79% (3). On the basis of Health Insurance Plan of Greater New York and Breast Cancer Detection Demonstration Project data, the estimated lead-time (the time between detection on mammography and when the lesion is clinically detectable) for invasive breast cancer is 1.7 years (range: 0.4-3 years), which suggests that the optimal screening interval would be no more than 1.7 years. The majority of the screening mammography clinical trials had an upper age limit criteria ranging from 64-74 years. Medical co-morbidity and life expectancy should be considered in a breast cancer-screening program for women aged 75 years or older because the benefit-to-risk ratio of screening mammography continues to shift adversely with advancing age. A consensus of recommendations does not exist. However, meta-analysis concluded that screening mammography in women aged 70-79 years is moderately cost-effective and yields a small increase in life expectancy (3). Initial concerns about the risk of radiation (eg, induction of breast cancer by radiation) have largely been allayed by improvements in mammography technique, technology, and clinical experience. False-positive mammograms (ie, those with perceived abnormalities requiring further evaluation to verify that the lesion is not cancer) are a continuing concern. False-positive screening mammograms require diagnostic mammography with supplementary views, ultrasonography, and even biopsy in 20-30% of cases in an attempt to reach an accurate diagnosis. Psychological consequences of screening mammography have been identified and reviewed and these psychological, behavioral and quality-of-life issues seem to be intrinsic to the fear of breast cancer. Ultrasonography is an established adjunct to mammography in the imaging evaluation. It is useful in evaluating inconclusive mammographic findings, in evaluating young patients and other women with dense breast tissue, in guiding tissue core-needle biopsy and other biopsy techniques, and in differentiating a cyst from a solid mass. Fine-needle aspiration cytology, particularly in Europe, has replaced excisional biopsy for evaluation of mammographic abnormalities. Many times a definitive diagnosis is not possible, mainly because of its inability to differentiate invasive from in-situ carcinoma. Stereotactically guided core needle biopsy (CNB), which uses a large-bore needle, appears to have several advantages over fine-needle aspiration cytology. CNB is less expensive than excisional biopsy, results in less morbidity, and leaves no noticeable scar. Certain benign lesions have a relatively high incidence of coexisting carcinoma, and the CNB with its small volume of tissue may not be adequate to rule out cancer. Magnetic resonance imaging can be a useful adjunct to diagnostic mammography, but cost, duration of the examination, and injection of contrast material prohibits its use as a routine, population-based screening technique. Color Doppler ultrasonography, computer-aided detection, positron emission tomography, scinti-mammography, step-oblique mammography, and thermography have shown promise in specific, selected situations but remain under clinical investigation and have not proposed as effective screening techniques. Although current imaging studies have proved effective in identifying malignancy, they have a limited ability to detect in-situ lesions. Ductoscopy is invasive, and requires sedation and anesthesia. Various procedures are available to obtain ductal cells for evaluation, such as collection of nipple aspirate fluid (NAF), random periareolar fine needle aspiration, and ductal-lavage. The average breast nipple contains five to nine milk pores. Each pore extends into a central duct, which has lateral lobular unit, where milk is produced during lactation. The lobules end at the terminal ductal lobular unit, where milk is produced during lactation. Research has suggested that cellular atypia is significant biomarker that precedes most malignant lesions, and is highly predictive of breast cancer development. Given the theory that active ducts are more likely to yield cells, patients should also be selected based on their ability to yield nipple aspiration fluid (NAF) with gentle aspiration suction. This process also identifies the orifice of fluid-yielding ducts for lavage. To perform ductal lavage, topical anesthetic is applied, and the ductal orifice cannulated with a flexible micro-catheter. Anesthetic is then often infused through the catheter to numb the duct. Saline is infused to rinse through the ductal lobular unit, and then the fluid is collected. The specimen is prepared with standard Pap staining, and evaluated cytologically. Ductal lavage should be used in conjunction with, and not in place of, clinical breast examination and mammography. The American Cancer Society (ACS) believes that ductal lavage may be useful in helping the small group of high-risk patients to further assess their risk and make decisions regarding tamoxifen, hormone therapy, and surgical prophylaxis. The American Society of Breast Surgeons has also issued an official statement supporting ductal lavage. Cells have two copies - BRCA1 and BRCA2, and they are tumor suppressor genes. Protein products of these genes interact with each other in a pathway that repairs damaged DNA. BRCA1 - 2 mutations increase the risk of cancer more than other factors and these mutations increase the risk of early-onset breast cancer. Despite an estimated 50-80% lifetime risk of breast cancer for those who carry the BRCA1 or BRCA2 mutation, few have advised any changes in the population-based guidelines for screening mammography in this high-risk group. Acknowledging that no surveillance data or related clinical trials are available for BRCA1 or BRCA2 carriers, the Cancer Genetic Studies Consortium has offered Level-III provisional recommendations of 1) education regarding monthly breast self-examination, 2) annual or semiannual clinical breast self-examination beginning at age 25-35 years, and 3) annual mammography beginning at age 25-35 years (4). The incidence of BRCA1 & 2 Mutations in Ashkenazi population is 1/40 and in non-Ashkenazi population it is 1/400 to 1/800. Should all Ashkenazi women be screened for BRCA founder mutations? The arguments in favor are: it identifies mutation carriers early, leading to prevention or early diagnosis. Genetic discrimination is unlikely to be a concern. The arguments against are: false negative if only founder mutations are screened and false sense of security for those who test negative and carry baseline risk of cancer. Insurance coverage is widely available for genetic testing. 97% of completed insurance claims from 2000-2001 were reimbursed at an average of 94% reimbursement. Many insurance carriers have established guidelines to determine eligibility for counseling and testing. Medicare now pays for testing. Benefits are: it provides risk information for individuals and families, provides information useful in healthcare and results can alleviate uncertainty and anxiety. Limitations are: a negative result is most definitive if there is a known mutation in the family. Some genetic variants are of unknown clinical significance. The search for ways to prevent breast cancer has been ongoing for many years. Tamoxifen (a triphenylethylene), which is an anti-estrogen although it is chemically related to an estrogen, was really the first selective estrogen receptor modulator. There has been more than a quarter of a century experience with tamoxifen as an adjuvant in patients with breast cancer. Because data suggested considerable protection in the development of contralateral breast cancer in women receiving tamoxifen vs. those not receiving tamoxifen, a large breast cancer prevention trial (P-1) was begun in 1992 (National Surgical Adjuvant Breast Project [NSABP]/NCL). The Gail model, an algorithm for estimating breast cancer risk, determines high risk. The Gail model is intended for women in a program of annual screening with mammography. This model predicts the risk for breast cancer in 5 years or life expectancy. More recently, other selective estrogen-receptor modulators have become available for evaluation; raloxifene is the one most extensively studied. This drug was primarily used to determine the prevention of postmenopausal osteoporosis in participants of the Multiple Outcomes of Raloxifene Evaluation (MORE) who were treated for 3 years. The risk of invasive breast cancer was 76% lower in the raloxifene group than in the placebo group. This was attributed to a 90% decrease in the development of estrogen-receptor-positive tumors. These results have led to the Study of Tamoxifen and Raloxifene (STAR) trial for evaluation of these drugs in the prevention of invasive breast cancer in postmenopausal women who are at high risk for this disease. Atypical Hyperplasia: it occurs early in the continuum of histologic changes that constitute the process of transformation to the malignant phenotype. A histologic or cytologic finding of atypia is a strong risk factor for developing breast cancer. The risk more than doubles for women with a family history of breast cancer (relative risk: atypia alone, ~5; atypia + family history, 11-18), and approximately 40% of these women will develop breast cancer in their lifetime. Atypical hyperplasia can be used as a risk estimator of developing breast cancer, since most subsequent breast cancers occur within 5 years of diagnosis (6). Mammographic Breast Density: the density of woman's breast can be evaluated on a routine mammogram and has been shown to correlate with breast cancer risk. The mammographic parenchymal pattern (breast density) refers to the amount of connective and glandular tissue relative to fat tissue in the breast. Since breast cancers are also composed of dense tissue, high breast density can mask tumors on mammography, preventing their early detection. High breast density is also a risk factor for breast cancer but may be independent of estrogen exposure or estrogen-receptor status (7). Thus, the increased risk of breast cancer associated with breast density can also result from non-estrogenic factors, such as levels of insulin-like growth factor, diet, and/or genetic and environmental factors. Breast density varies significantly among women and can have high (~63%) heritability. Computerized texture analysis of mammographic images demonstrated that the parenchymal patterns in high-risk women who carry BRCA1 or BRCA2 gene mutations were dense, coarser, and had lower contrast with less local variation than those of women at low risk for developing breast cancer (8). Histologic and cytologic analysis for breast cancer risk: detection of abnormal cells in the breast has been shown to be a risk factor for breast cancer. There are a variety of different methods to obtain a sample from the breast. Traditional methods of collecting cells for histologic or cytologic analysis -- fine needle aspiration (FNA), nipple aspirates, core needle biopsy, and surgical biopsy -- all have limitations (9). Random sampling with FNA, for example, is an invasive procedure and frequently yields insufficient tissue for analysis, particularly in older women. Similarly, nipple aspirates frequently contain insufficient cells. Core needle biopsy and surgical biopsy yield ample cells but are invasive procedures that would not be performed only for risk assessment. A newer, less invasive method used for histologic or cytologic analysis is ductal lavage, which is a minimally invasive procedure that extracts ductal fluid using a topical anesthetic and can be performed in outpatient settings. Possible candidates for ductal lavage are women who are estimated by the Gail model to be at increased risk for developing breast cancer. Breast Imaging: it has been a tool for predicting the likelihood of diagnosing breast cancer. The Breast Imaging Reporting and Data System (BI-RADS), developed by the American College of Radiology, standardized the assessment of images to aid in determining appropriate follow up. Suspicious abnormalities -- lesions that require intervention (eg, biopsy) but are not necessarily malignant -- are assigned to category 4. In a study performed to evaluate the use of the Gail model as an adjunct to the BI-RADS score, women having category 4 lesions and an elevated 5-year Gail risk (>1.7%) had significantly more malignant findings at biopsy than those with category 4 lesions and low Gail risk (42% vs 21%, p<.0001; relative risk 1.94). The investigators theorized that the method may be most useful when applied to BI-RADS category 3 lesions, but the number of women in this category was too small for analysis (10). The use of breast imaging as an adjunct to the Gail model for enhancing risk assessment requires further analysis. According to behavior theories, a perception of higher risk would increase the use of screening, while a perception of lower risk would have the opposite effect. Although this is true in many cases, the issue is complex. Studies found that reducing a woman's perceived risk did not adversely affect her intention to obtain a mammogram and may have increased compliance with screening recommendations. Breast cancer-related psychological distress (eg, severe worry) has been associated with poor compliance with screening recommendations, even among women identified to be at high risk, whereas moderate worry was associated with higher likelihood of screening. It has been found that the relationship between worry about breast cancer risk and getting mammograms is associated with an inverted U-shaped pattern. Women with moderate levels of worry were more likely to have mammograms regularly than those worry level was either very low or very high. The investigators theorized that severe worry triggers denial and avoidance behavior, whereas moderate concern vigilance and motivation to use screening methods (11). Genetic counseling can provide critical assessment of the family history to help determine the likelihood of an inherited cancer susceptibility syndrome and the appropriateness of genetic testing. The subsequent clinical recommendations for mutation carrier need to take into account the patient's age, desire for future childbearing, and other medical history when prescribing screening interventions or prophylactic surgery (12). Practical applications of genetic testing for cancer susceptibility have the ability to reduce the burden of hereditary cancers by saving lives, decreasing medical morbidities, and reducing psychological stress. Hereditary breast and ovarian cancer is an inherited cancer-susceptibility syndrome. The hallmarks of this syndrome are multiple family members with breast cancer or ovarian cancer or both, the presence of both breast cancer and ovarian cancer in a single individual, and early age of breast cancer onset (often before age 50). The vast majority of hereditary breast and ovarian cancer is due to an alteration or mutation in either the BRCA1 or BRCA2 genes. These mutations can be inherited from either mother or father. Current strategies to reduce risk of developing breast cancer in women with known deleterious BRCA mutations include surveillance, chemoprevention, and surgery. Recommended surveillance includes clinical breast examination semiannually as well as both annual mammography and annual breast magnetic resonance imaging screening beginning at age 25 years or sooner based on earliest age onset in the family (14). Magnetic resonance imaging is more sensitive for the detection of breast cancer than mammography, and the combination of magnetic resonance imaging, mammography, and clinical breast examination has the highest sensitivity for the detection of breast cancer in high-risk BRCA mutation carriers. Risk reducing salpingo-oophorectomy reduces the risk of breast cancer by 40-70% (13). This protection likely occurs only if patients are premenopausal at the time of risk-reducing salpingo-oophorectomy. In addition, studies have shown BRCA1 mutation carriers may have less of a protective effect from risk-reducing salpingo-oophorectomy on breast cancer risk than BRCA2 mutation carriers (14). It is important for high-risk individuals to stay in contact with clinicians experienced in the care of women at increased risk, given rapidly developing research and refinements in testing technology. For example, a test for large rearrangements in the BRCA1 and BRCA2 genes has been developed that may help to identify mutations in a small percentage of the high-risk families who previously tested negative for these genes. A genetic risk assessment is recommended for patients with a greater than an approximately 20-25% chance of having an inherited predisposition to breast cancer and ovarian cancer. The Gail model is a validated tool for Caucasian women that estimate a woman's absolute risk of developing invasive breast cancer in the next 5 years based on the risk factors discussed above. There is no single tool that can predict precisely which women will get breast cancer. National Cancer Institute breast cancer risk assessment tool and risk calculator are available at: http://www.cancer.gov/bcrisktool/ Women aged 40-49 years should have screening mammography every 1-2 year. Women aged 50 and older should have annual mammography. Despite a lack of definitive data for or against breast self-examination, breast self-examination (BSE) has the potential to detect palpable breast cancer and should be recommended. All women should have clinical breast examinations annually as part of the physical examination. When a non-palpable mass is perceived on screening mammography, the patient should be referred to a professional experienced in the diagnosis of breast cancer so that a complete imaging work-up can be performed. Mammography is useful in evaluating women who suspect they have a breast mass that the clinician cannot palpate. In such cases, focused ultrasound imaging may be a component of the complete imaging evaluation at any age. A non-palpable mass may require needle localization biopsy or stereotactic biopsy. Women may benefit from genetic counseling and testing if their family history, including age at diagnosis and types of all cancers (in any relatives, female and male), suggests a possible autosomal dominant cancer pattern or if a BRCA mutation has been discovered in their family. Genetic counseling will address the risks and benefits of the test. |