Breast Cancer Risk AssessmentWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). Breast cancer biology is a topic of intensive research, and there have been huge breakthroughs in recent years. Scientific insight into estrogen-receptor biology has led to major advances in estrogen-targeted prevention and chemotherapy. Increased breast density has emerged as a dominant, detectable, and modifiable risk factor for subsequent development of breast cancer in women. Factors that increase a more mature woman's risk of cancer, such as breast density, can be identified in midlife. Enhanced imaging techniques, such as digital mammography and MRI, can be deployed effectively in smaller group of high-risk women to arrive at a more balanced and appropriate ratio of not only cost-effectiveness but also harm to benefit. It is prudent not to rely on mammography to "solve" the problem of early detection of breast cancer. Breast cancer risk assessment is a routine aspect of women's healthcare, and 3 models are available to help clinicians with this task: Gail, Claus, and Tyrer-Cuzick. A fourth -- and increasingly common tool is genetic testing, which can be an important piece of genetic risk assessment for some individuals. The models available to clinicians to assess a person's risk of breast cancer can produce widely varying estimates, and clinicians must understand the limitations of each model to choose the most appropriate model for their patient. The purpose of this document is to discuss clinical application of the various tools available to assess a woman's risk for breast cancer and apply in clinical practice. This review also provides a comprehensive examination of the importance of breast density as a dominant risk factor for the development of breast cancer, highlighting the role that genetics and hormones play in maintaining breast density in postmenopausal women. Future research can be directed toward the detection of preexisting conditions (such as gene mutations) that put women at risk for highly aggressive cancers. Recommendations for genetic testing are reviewed here, with a particular focus on the components of genetic counseling, identifying individuals for testing, and interpreting test results. Background:Lifetime risk of breast cancer for the average woman is approximately 12% (1). Risk factors for breast cancer are well described. The use of exogenous hormones (estrogen and progesterone) are known to increase risk, and endogenous hormonal factors such as ages at menarche, menopause, and first childbirth are associated with a slight, but significant, increase in breast cancer risk. Environmental factors, such as diet and alcohol use, although not well understood, also are associated with breast cancer risk. Having had a breast biopsy, and especially a biopsy that demonstrated atypical hyperplasia or lobular neoplasia, is associated with a significant risk of breast cancer (2). A positive family history of cancer also suggests a genetic component to a person's risk. Many of these factors have been incorporated into the models discussed below. The breast is composed of three main tissues -- fat, fibrous/connective tissue and epithelial tissue. Differences in the relative amount of these tissues result in variability between individuals in the radiologic appearance of the breast on the mammogram. Fat is radiolucent and appears dark on the mammogram, whereas epithelium and connective tissue are radio-dense and appear light on the mammogram. Mammographic breast density (MBD) reflects the appearance of the breast on the mammogram and indicates the relative proportion of radiographically dense, ie, white or light areas of the breast. Increased breast density is associated with a higher risk of breast cancer independent of the increased difficulty of reading a mammogram (3). White women have greater risk of developing breast cancer than black women (although black women diagnosed with breast cancer are more likely to die of the disease). Other risk factors for breast cancer have been identified or proposed but are not included in the Breast Cancer Risk Assessment Tool for several reasons: because evidence that these factors contribute to breast cancer risk is not conclusive, because researchers cannot determine whether these factors add useful information to factors already in the model, or because data on other risk factors was not available in the research data used to develop the model. Such risk factors include: age at menopause, use of birth control pills, high body mass index, a high-fat diet, alcohol, radiation exposure, and environmental pollutants. Recently published research indicates that breast tissue density, measured from mammograms, can add useful information, but risk models with breast tissue density measurement still need to be validated with additional independent studies (3). Research also indicates that other risk factors, such as use of hormone therapy, might improve the tool. Introduction:The Breast Cancer Risk Assessment Tool was developed for women in the United States population age 35 years or older (4). It should not be used for women with a previous diagnosis of breast cancer, women exposed to breast radiation for treatment of Hodgkin Lymphoma, or women who reside in, or recently migrated from, regions with low breast cancer risk, such as rural China or Japan. More accurate methods to project risk may be available for women with certain rare identified mutations, such as alterations in the breast cancer susceptibility genes BRCA1 and BRCA2. The Breast Cancer Risk Assessment Tool was developed and has been validated in populations consisting mainly of non-Hispanic white women. More research is needed to validate or refine the model for other racial and ethnic groups. The Breast Cancer Risk Assessment Tool is a computer program that was developed by scientists at the National Cancer Institute and the National Surgical Adjuvant Breast and Bowel Project (NSABP) to assist health care providers in discussing breast cancer risk with their female patients. The tool allows a health professional to project a woman's individual estimate of breast cancer risk over a 5-year period of time and over her lifetime and compares the woman's risk calculation with the average risk for a woman of the same age. The Breast Cancer Risk Assessment Tool can be found at: http://www.cancer.gov/bcrisktool. Understanding Risk Factors:Although each of these models provides a short- and long-term risk, the use of lifetime risk allows comparison of risk estimates generated by each model. An understanding of each model's limitations will allow the clinician to use the most appropriate model for assessing risk and counseling a woman in a given situation. For information to help your patients understand cancer risk visit http://understandingrisk.cancer.gov. This interactive Web site will help your patients make informed decisions about how to lower their risk. The Gail Model:This model incorporates (5):
Relative Risk of Developing Breast Cancer
For women with 0 or 1 affected relative, risks increase with age at first live birth. For women with 2 or more first degree relatives, risks decrease with age at first live birth. This model does not account for family history in relatives other than mother and sister. Importantly, the Gail model fails to account for paternal family history of breast cancer. This model predicts the risk for breast cancer in 5 years or life expectancy. The new model can be used to project risk over 5, 10, 20 and 30 year intervals. The new model predicted higher risks than the previous model in women with high breast density, and previous analyses indicated that the new model had modestly higher accuracy (6). Independent validation studies are needed before this model should be used for counseling, and before making a permanent change to the Breast Cancer Risk Assessment Tool. The Claus Model:This factors in breast cancer history in up to 2 first- and/or second-degree maternal or paternal relatives and the age at which these persons were diagnosed with breast cancer (7). This information is used to calculate risk based on present age and lifetime risk. Lifetime risk of breast cancer based on family history: Claus Model (8)
The Tyrer-Cuzick Model:Presently, a research tool, attempts to address the limitations of the previous models by including a variety of risk factors (9). The model uses age, body mass index (BMI), hormonal and reproductive factors, breast disease, and an extensive family history to calculate personal breast cancer risk. One study showed that this model produced a higher ratio of expected to observe cases of breast cancer than did the Gail and Claus models, indicating that it may produce a more accurate assessment of risk (10). This model has not yet been validated and should therefore, be used cautiously in a clinical setting. Hereditary Breast and Ovarian Cancer SyndromeApproximately 5% to 10% of breast cancer are hereditary and 15% to 20% of breast cancer cases likely to have familial component and result from gene-environment interactions (11). Although BRCA1 and BRCA2 are the genes most commonly screened for, they are probably not the only genes implicated in familial breast cancer: in as many as 70% of cases, testing the person in a family most likely to have hereditary cancer yields a negative result. BRCA1 and BRCA2 mutations are associated with a 45% to 85% lifetime risk of breast cancer, with average age of onset of 43 years for BRCA1 and 45 years for BRCA2 (12). Both BRCA1 and BRCA2 are associated with a >50% risk of a second primary breast cancer. BRCA1 is associated with a 45% lifetime risk of ovarian cancer, whereas BRCA2 has half that risk (13). Researchers have identified regions with BRCA2 that are associated with higher rates of ovarian cancer; in clinical practice, however, all deleterious mutations within each gene are given similar rates of cancer risk. Families with BRCA1 or BRCA2 mutations have an increased risk of prostate cancer. Families with BRCA2 mutations are at risk for male breast cancers as well as pancreatic, laryngeal, and gallbladder/bile duct cancers, and melanoma (13). The prevalence of BRCA1 and BRCA2 mutations is estimated to be between 1 in 300 to 800 persons of European heritage and 1 in 40 persons of Ashkenazi Jewish heritage. Genetic testing for BRCA1 and BRCA2, which has been clinically available for years, involves evaluation of the entire coding region of each gene in search of large deletions. Interpretation of genetic testing can be complicated, especially if the significance of the genetic alteration identified is uncertain. In such cases the mutation is labeled a "variant of uncertain significance" and cannot be used for testing of other family members. In 2009, American College of Obstetricians and Gynecologists (ACOG) published a recommendation that women with a 20% to 25% chance of having a BRCA1 or BRCA2 mutation undergo genetic risk assessment and be considered for genetic testing (14). ACOG further identified women who have a 5% to 10% risk of BRCA1 or BRCA2 mutation as potential candidates for genetic risk assessment. 2009 ACOG recommendations for genetic testing in women are:
The Value of A Full Pedigree Analysis:Given that BRCA1 and BRCA2 are associated with a variety of cancers, patients should be asked about all known cancers and age at diagnosis among relatives. Patients should also be asked specifically about cancers present in second- and third-degree relatives (grandparents, aunts, uncles, and first cousins), as this information may be helpful in identifying an autosomal dominant pattern. It is important to capture parental family history and to ask about adoption. Some situations may obscure a family history of hereditary breast and ovarian cancer. Relatives who have had a hysterectomy or oophorectomy have significantly reduced their chances of ever having breast or ovarian cancer. Family size and gender distribution will also affect the prevalence of hereditary cancer within a family, eg, a very small family with few women will have very few members who might express the phenotypes (15). Lastly, it is often helpful to review medical records of family members to develop a family history of hereditary cancer, since not all gynecologic cancers are equal in this regard. Interpreting Genetic Results:A key principle in genetic testing for hereditary cancer is to test the person in the family who is most likely to have that cancer. In some families, that person is a relative who has been given a diagnosis of breast cancer. Testing that person can determine whether a mutation exists in family, establishing a background against which to interpret the genetic test results of relatives. If a family is known to have a genetic mutation, then interpreting the test of another relative is straightforward: A positive test result for the same mutation is associated with increased cancer risk. A negative result means that the patient is at average cancer risk; her family history is related to the presence of a mutation that she has not inherited. Patient management after inconclusive results: The following scenarios illustrate the difficulty of interpreting negative BRCA1/2 results in a family with a history of cancer, but no known mutation. Scenario 1: Patient A is diagnosed with breast cancer at age 41 years. Her sister was diagnosed with ovarian cancer at age 52 years. Patient A is the person in this family most likely to have a genetic mutation, but testing shows that she is BRCA1 and BRCA2 negative. Interpretation: This test does not rule out hereditary cancer in this family. It is also not possible to rule out the risk of ovarian cancer for patient A, given her family history. Thus, the genetic test is inconclusive. The risk assessment and management plan for Patient A should be based on her family history. 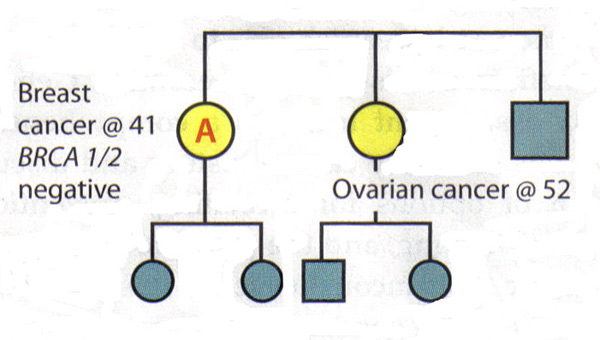 Scenario 2: Patient B's late mother and maternal aunt were diagnosed with breast cancer and ovarian cancers, respectively. Patient B requests genetic testing for hereditary cancer -- the first in the family to do so -- and finds that she is BRCA1 and BRCA2 negative. Interpretation: By itself, this result is inconclusive. If her mother had a mutation, then it would be possible to conclude from the negative results that Patient B is at average risk. However, since her mother's and aunt's status is unknown and it is no longer possible to test them, the interpretation of this result is unclear. There may not be a mutation in the family or there may be a mutation that Patient B did not inherit. Alternatively, the test may not have been done correctly or there could be a mutation in a gene other than BRCA1 or BRCA2. Therefore, Patient B should be managed according to her family history. 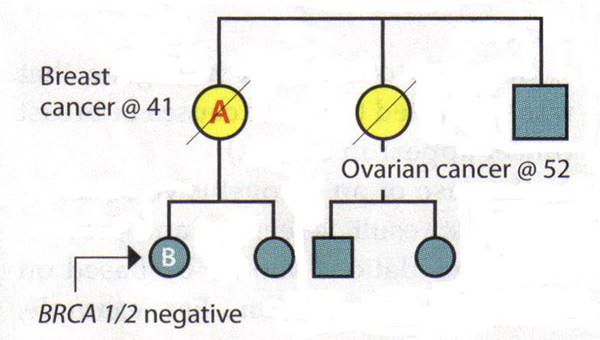 Causes of Hereditary Breast Cancer:The negative results in an individual could mean several things:
Mammographic Breast Density (MBD) and Breast Cancer RiskMammographic breast density (MBD) reflects the appearance of the breast on the mammogram and indicates the relative proportion of radiographically dense, ie, white or light areas of the breast. Several classification schemes have been used to categorize MBD over the decades. The earliest categorization was Wolfe's parenchymal pattern that classified the extent and type of density into 4 categories (16):
Currently, the most common clinical measure of density is the Breast Imaging Reporting and Data Systems (BI-RADS) density method, proposed by the American College of Radiology (3)(17). BI-RADS density is a subjective measure used by radiologists to classify a mammogram as follows:
Computer assisted methods are used to estimate quantitative measures of MBD, including percent density (percentage of the overall breast showing dense tissue; PD), absolute dense area, and non-dense area (3)(16). MBD and Breast Cancer Risk:Elevated MBD is considered one of the strongest risk factors for breast cancer regardless of whether it is assessed as a categorical or quantitative measure (16). Women in the highest categories of breast density have a 4- to 6-fold increased breast cancer risk compared with women in the lowest categories. MBD has been shown to be a stronger risk factor for breast cancer than any others except age and genetic mutations (16). The association between MBD and breast cancer has been seen both in older and younger women undergoing screening mammograms, as well as in Caucasian and non-Caucasian population. Hormonal Influence of MBD:There is strong and consistent evidence that MBD is influenced by hormonal factors such as menopause, age at menarche, parity, age at first birth, and use of exogenous hormones (18). The influence of exogenous hormones on MBD is best illustrated by positive associations of MBD with postmenopausal hormone (PMH) therapy and inverse associations with tamoxifen (18). These associations are important because they suggest inter-individual variability in response to hormone therapies manifested in MBD changes, which may translate into differential breast cancer risk. With estrogen depletion during menopause, the breast glandular tissue undergoes regression. This is also reflected by a decrease in MBD during and after menopause. When the process is interrupted by PMH, studies evaluating MBD by quantitative measures suggest that these hormones are associated with an absolute increase of 3% to 6% in MBD (19). In the Norwegian Breast Cancer Screening Program (19), current users of PMH therapy had a significantly higher mean percent MBD than never users for never users (p for trend <.001). Similarly, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial, which examined the association between PMH and MBD, also saw increases with combined hormone therapy (20). In the Women's Health Initiative (WHI) study, women were randomized to receive daily combined conjugated equine estrogens (CEE, 0.625 mg) plus medroxyprogesterone acetate (MPA, 2.5 mg) or placebo. More than 75% of women on active PMH had an increase in MBD (18). Taken together, these studies suggest that estrogen-and-progestin combined therapies at conventional doses are associated with increased breast density, regardless of the pattern of progestin administration. And, importantly, the associations of these therapies with MBD change parallel the associations with breast cancer risk, such that there are increases in breast cancer with combination therapy but not with estrogen alone (21). Although lower dose regimens are hoped to have less influence on breast density, a recent study found no difference between the associations of conventional and low-dose hormone therapy with BMD (22). Stopping for 1 to 2 months was associated with only small decreases in MBD, and stopping therapy for this short period did not affect mammogram recall rates (22). 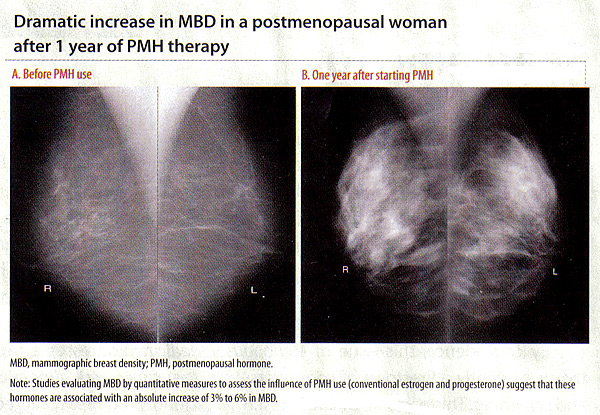 Influence of Tamoxifen, Raloxifene, and Aromatase Inhibitors on MBD:In view of the positive association between PMH and MBD, the logical question is whether tamoxifen, raloxifene or aromatase inhibitors reduce MBD. Tamoxifen: is a selective estrogen receptor modulator (SERM) that competitively binds to estrogen receptors and blocks estrogen synthesis. The antiestrogenic influence on the breast resulted in its use as adjuvant endocrine therapy for women with estrogen receptor-positive postmenopausal breast cancer. Tamoxifen has also been used as a chemopreventive agent to reduce the risk of breast cancer in women at high risk. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized clinical trial of high-risk women, 5 years of tamoxifen therapy was shown to reduce invasive breast cancer risk by 49% and non-invasive breast cancer risk by 50% compared with placebo (23). Several studies have examined the effects of tamoxifen on MBD using different estimation methods for MBD and study populations (ie, women with known breast cancer on adjuvant tamoxifen or women at high risk for breast cancer on tamoxifen for chemoprevention). These studies have consistently demonstrated that women on tamoxifen do experience a statistically significant reduction in MBD. Some of these studies noted that decreases in percent MBD occurred at a greater frequency in premenopausal than in postmenopausal women (24). Common to all of these studies was the fact that not all women on tamoxifen experienced a reduction in breast density with therapy; the proportion of women experiencing a reduction in density ranged from 21% to 80%. This was seen across studies with varying MBD estimation methods, populations (whether high-risk women or cancer cases), and as noted above, varying menopausal status. This suggests the hypothesis that decreases in MBD induced by tamoxifen in a portion of women have clinical significance, resulting in fewer incident cancers among high-risk women and fewer instances of breast cancer recurrence or contralateral events among cases. These women stand to benefit by remaining on tamoxifen. In contrast, those who see little reduction or even increases in MBD may be those who would benefit from alternative treatment approaches. Raloxifene: anther SERM agent has been used for chemoprevention of breast cancer due to the demonstrated reduction in the risk of invasive breast cancers after 5 years of therapy (23). Some studies have shown similar decreases in MBD among women on raloxifene vs placebo, where as others have shown small changes in MBD with raloxifene use (25). In a study of raloxifene and MBD assessed as volumetric breast density from full-field digital images, it is shown a small reduction in volumetric MBD in the raloxifene group (median -4.1%; 95% CI, -6.9%-2.1%), compared with an increase in MBD seen in low-dose PMH group (median 15.0%; 95% CI, 4.8%-28.6%; p<.0001). Aromatase inhibitor (AIs): block local synthesis of estrogen in extracts of human breast tumors and currently are the most efficacious endocrine therapy for estrogen receptor-positive postmenopausal breast cancer. Few studies have examined the AI, letrozole, and MBD, with mixed results. These include a study of 106 postmenopausal women who were randomized to either letrozole or placebo after 5 years of tamoxifen; this study found no difference between the 2 groups in change in MBD after 9 to 15 months (26). The inconsistent findings of studies of raloxifene and AIs with MBD may be related to the fact that these therapies are used only in postmenopausal women with lower baseline MBD; making small changes hard to detect. However, large studies with well-calibrated density measures and follow-up for breast cancer are needed to determine whether MBD can be used as a biomarker for these and other endocrine therapies. Clinical Application of MBD and Breast Cancer Risk:Studies have recently incorporated the BI-RADs and quantitative MBD measure into breast cancer risk prediction models, which has shown some improvement in risk prediction (improvement c-statistic by 0.01 to 0.06) (27). An enhanced model with an MBD measure is, therefore, preferable to the currently existing Gail model but remains poor for individualized risk. Also, it is important to recognize the clinical challenges associated with evaluating mammograms of women with increased MBD, including the need for repeat mammograms and breast biopsies and the difficulty of detecting clinically significant abnormalities. Given the associations between exogenous hormones and a change in MBD, a natural question is whether a change in MBD is a potential marker for risk. In other words, if women experience increased MBD with PMH use, are they at greater risk for breast cancer than women who decrease or maintain MBD while on PMH? Or if a woman on adjuvant tamoxifen decreases MBD, does this mean that she will have a reduced risk of recurrence compared with a woman who has no change in MBD while on tamoxifen? Most of the studies suggest that change in MBD could be a biomarker for breast cancer risk reduction for women undergoing chemoprevention strategies and could allow for earlier identification of women who would not benefit from tamoxifen therapy (28). Studies are also under way to examine the influence of change in MBD with PMH use on breast cancer risk, and results are expected soon. At this time, it is not clear whether the resulting increases and decreases in MBD with endocrine therapies simply change the ability to detect new breast cancers or relate to the pathophysiology of the breast cancer. Additional work is needed to improve our understanding of agents that contribute to change in MBD and their association with breast cancer. Also, the development of reproducible and well-calibrated density measures that can accurately measure MBD are needed to allow for comparability of MBD change across studies and therapy types (28). Summary:Cancer risk assessment is a comprehensive review of and discussion about a person's risk for cancer. This includes obtaining information regarding factors that may mitigate cancer risk (eg, use of hormone therapy, use of oral contraceptives, age at menarche and first childbirth). Other key components of the risk assessment include discussion of the genetic basis of cancer, family history of cancer, family history of cancer, likelihood of genetic syndrome, and options for screening and prevention. Genetic testing is a tool that can refine risk assessment. Before any genetic test is performed, the possible results and their implications for the individual and her family should be discussed. This expensive test has minimal value to a patient who is unwilling to share the results with her relatives. If genetic testing is appropriate, the genetic counselor or other healthcare provider may facilitate genetic testing by investigating insurance coverage, providing test results and follow-up care, and helping the patient inform at-risk relatives. Understanding variability in the response to endocrine therapies is important so that the most effective therapy can be administered to patients in timely manner. This includes the administration of exogenous estrogens to healthy women to reduce postmenopausal symptoms as well as breast cancer treatment for women with disease. MBD may contribute to our understanding of inter-individual variability in response to therapy. This information can then translate to the clinical setting to facilitate personalized decision-making regarding options for breast cancer treatment and risk-reduction strategies. References:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||