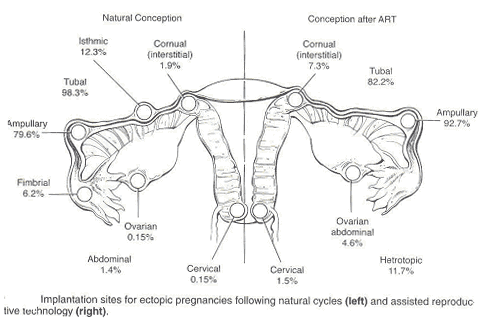
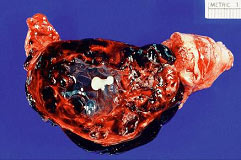
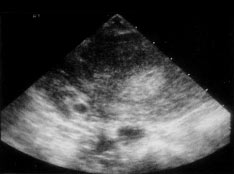
Ectopic PregnancyWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). Ectopic pregnancy is a condition in which an early embryo (fertilized egg) implants outside the normal site for implantation (uterus). Ruptured ectopic pregnancy is the leading cause of morbidity and mortality for women in the first trimester of pregnancy and accounted for 10.8% pregnancy related deaths in the United States. Women who present with pain and bleeding in the first trimester are at risk for ectopic pregnancy, a life threatening condition. Ectopic pregnancy is still the leading pregnancy-related cause of death in the first-trimester and accounts for 9-13% of all pregnancy related deaths. If undiagnosed and/or untreated, ectopic pregnancy may result in rupture of fallopian tube and massive intraperitoneal hemorrhage, accounting for about 9% of maternal pregnancy-related deaths in this country. The primary goal of accurate and early diagnosis of ectopic pregnancy is to limit morbidity and eliminate mortality resulting from this condition. If diagnosed early, at a clinically stable state, the patient with an ectopic pregnancy is likely candidate for either minimally invasive surgery or medical therapy. The purpose of the document is to diagnose early, and to understand conservative medical and surgical treatments that are now widely available for ectopic pregnancies. Methotrexate, a folinic acid antagonist, has been used to treat patients with small unruptured tubal pregnancies. Evidence, including risks benefits, about methotrexate as an alternative treatment for selected ectopic pregnancy is also discussed. Early detection may make it possible for some patients to receive medical therapy instead of surgery. It is estimated that about 2% of pregnancies in the United States are ectopic. In the year 1992, approximately 2% of all pregnancies in the United States were diagnosed as ectopic (1). Recent studies indicate that the incidence of ectopic pregnancy has increased six-fold over the last 25 years. Hospital admissions for the condition increased dramatically from 17,800 in 1970 to 88,400 by 1992 (2). The prevalence of ectopic pregnancy appears to be rising, in part because of earlier and more accurate diagnosis of pregnancies. Further, an increased incidence of sexually transmitted infections, earlier diagnosis of pelvic inflammatory disease resulting in tubal damage but not complete blockage, and the rise in the number of ectopic pregnancies resulting from assisted reproductive technologies (ART) may account for the overall increase. The incidence of tubal pregnancy after oocyte retrieval/embryo transfer may be as high as 4.5%, although this may be due to already existing tubal pathology in these patients and not solely to ART intervention. The incidence of heterotopic pregnancy is now believed to be about 1:4,000 in the general population and 1:100 in in-vitro fertilization (IVF) pregnancies -- much higher than the originally described prevalence of 1:30,000 in the late 1940s (3). The cost of treatment for the 58,000 women needing hospitalization for ectopic pregnancy in just one year, 2002, based on U.S. health care statistics, was $ 1.1 billion. Considering that many more women are now treated with non-invasive surgery and/or on an outpatient basis, it is difficult to estimate the current number of annual ectopic pregnancies in the United States, as well as the cost of treating the condition. Early detection and treatment of ectopic pregnancy can lead to reduced morbidity and mortality, preservation of fertility and substantial cost savings to the patient, health systems and society (5). Counseling of high-risk patients prior to conception and early screening of patients with heightened risk of ectopic pregnancy can significantly affect patient prognosis and medical costs. As in naturally occurring ectopic pregnancies, the fallopian tube is the most common site for ectopic pregnancies following in-vitro fertilization (IVF). The most important predisposing factor for ectopic pregnancy in patients undergoing IVF is tubal pathology. Ectopic pregnancies are four times higher in patients with tubal-factor infertility compared to patients with normal tubes (3). The increasing use of ovulation induction agents that increase the chance of twining and may cause hormonal fluctuations affecting tubal motility, and also due to the invasive nature of assisted reproductive technology (ART), may be responsible in the increase incidence of ectopic pregnancy. Fallopian tube: This is the most common site of ectopic implantation, accounting for 97% of all ectopic pregnancies. Blockage of tubes or damage to tubal mucosa (causing decreased tubal motility) can impede the passage of the embryo to the uterus and results in the implantation of the embryo in the lining of the tube. The tubes cannot support the growing embryo, and may eventually rupture and bleed, resulting in a potentially catastrophic situation. The distribution of ectopic sites within the fallopian tubes is: ampullary (80%); isthmic (10%); cornual/interstitial (2%); fimbrial (5%). A unique factor for interstitial pregnancy is previous salpingectomy, present in about 25% of patients. Almost all cases of cornual/interstitial pregnancies are diagnosed after the patient is symptomatic. Abdominal/Peritoneal pregnancies: The incidence of abdominal pregnancy is estimated at 1 in 8,000 births, and abdominal pregnancy represents 1.4% of ectopic pregnancies. The prognosis is poor, with an estimated maternal mortality rate of 5.1 per 1,000 cases. The risk of dying from an abdominal pregnancy is 7.7 times higher than from other forms of ectopic pregnancy. Abdominal pregnancies can be categorized as primary or secondary. Primary abdominal pregnancies are rare and occur as a result of primary peritoneal implantation. They usually abort early in the first trimester due to hemorrhagic disruption of the implantation site and hemoperitoneum. Secondary abdominal pregnancies occur with re-implantation after a partial tubal abortion or intraligamentary extension. Historical criteria to distinguish between primary and secondary abdominal pregnancies are moot, because treatment is directed by clinical presentation (4). Other rare sites include ovary and cervix (1.6%). 90% of ovarian pregnancies are associated with IUD use. As in tubal pregnancy, inflammatory disease was present in 45.8% of ovarian pregnancies, unlike tubal pregnancies; there are no reports of recurrent ovarian pregnancies. The incidence of cervical pregnancy ranges from 1 in 2,500 to 1 in 12,422. The most common predisposing factor for cervical pregnancy is a prior dilatation and curettage, present in 68.6% of patients; other predisposing factors are previous cesarean delivery and IVF (4). Heterotopic pregnancy: It is the coexistence of an intrauterine and ectopic gestation. It occurs in 1 in 3,889 to 1 in 6,778 pregnancies (1). Simultaneous existence of intra- and extrauterine pregnancies poses several diagnostic pitfalls. Systemic methotrexate is contraindicated if a viable intrauterine pregnancy is present. If patient is hemodynamically unstable and exploratory laparotomy is warranted. Women with pre-existing tubal damage are more likely to develop an ectopic pregnancy. In fact, 50 percent of ectopic pregnancies are associated with some degree of tubal disease. A large percent of these women have no risk factors, yet may end up with an ectopic pregnancy. Some of these risk factors are (5,6,11): Ectopic pregnancy is frequently misdiagnosed, often with potentially life threatening consequences. The mean gestational age at time of diagnosis of an ectopic pregnancy is 7 weeks ± 2 weeks. The rupture of an ectopic pregnancy is a true medical emergency, and reaching an early diagnosis is especially critical. Ectopic pregnancy must be considered as a differential diagnosis in all women of reproductive age with vaginal bleeding and lower abdominal pain. The basis for definitive diagnosis is the exclusion of an intra-uterine pregnancy, rather than the proof of the existence of an ectopic. Since the signs and symptoms of ectopic pregnancy vary a lot, a high index of suspicion is essential to reach an early diagnosis. Some possible symptoms of ectopic pregnancy are listed below, though the specific presentation in an individual may vary. The most common symptoms are: late, delayed or abnormal menses (65%); abdominal or pelvic pain and cramping (98%); vaginal bleeding (80%); adnexal tenderness, pain after sex (54%); internal bleeding -- faint or giddy feeling, shoulder pain, low grade fever, palpitations, tiredness, collapse (7). Clinical examination is very rarely sufficient to diagnose an ectopic pregnancy, especially in the community or primary health center setting. Though physical examination is unreliable, typical findings may include the following: vaginal bleeding -- this is a suggestive finding, but may be mild or absent. Abdominal pain -- a frequent finding, this is strongly indicative if severe but may be moderate, mild or even absent in 9% of the cases. Tachycardia, hypotension, low grade fever -- present with severe pain, rupture and internal hemorrhage. Marked abdominal tenderness, especially rebound tenderness, with guarding suggests a diagnosis of ruptured or bleeding ectopic pregnancy. Internal examination (preferred to be done in a hospital setting) may show an adnexal mass (in 50% cases), tenderness, pain on moving cervix, and an enlarged uterus. Serial quantitative levels of the beta subunit of human chorionic gonadotropin (ß-hCG) can be used in combination with transvaginal ultrasonography and, in some cases suction curettage and serum progesterone measurements to differentiate failed intrauterine pregnancy, threatened abortion, and intrauterine or ectopic pregnancies. A presumptive diagnosis of unruptured tubal ectopic pregnancy is required before medical management can be considered. Beta Subunit of Human Chorionic Gonadotropin (hCG): The mean plasma concentration of human chorionic gonadotropin (hCG) is significantly lower for an ectopic pregnancy than for a viable intrauterine pregnancy, but there is no definitive laboratory level permitting distinction between the two. Urine pregnancy test helps establish a pregnancy, but can miss a very early ectopic (lower limit of detection: 50 mIU/mL hCG), hence a quantitative serum hCG (sensitive to 5 mIU/ml hCG) is necessary. Most reports and clinicians have found serial hCG testing useful in the early diagnosis of ectopic pregnancy. The rate of hCG doubling decreases from every 1.4-1.5 days in early pregnancy to every 3.3-3.5 days at 6-7 weeks of gestation, at which point the reliability of serial testing may be diminished (8). Ultrasonography: A trans-vaginal ultrasound is far more sensitive than trans-abdominal ultrasound in detecting the presence of an ectopic pregnancy. The gestational sac appears first as a 'double decidual sign' with a thick echogenic rim. The yolk sac appears at 5 weeks as a bright echogenic rim, sonolucent center and fetal pole. Cardiac activity appears at 6 weeks gestational age. Blood or fluid may be present in the cul de sac, indicating a bleeding ectopic. Ultrasound examinations with serum quantitative hCG correlation: hCG less than 1,500 mIU/mL - almost half of all patients who present with a suspected ectopic can not be definitively diagnosed with ectopic pregnancy at initial presentation. This may be because, at low levels of hCG (less than 1,500 mIU/mL), ultrasound examination may not show evidence of a gestational sac. Ultrasound has only 30 -- 40% accuracy when predicting an intrauterine pregnancy, miscarriage or ectopic pregnancy below the 'discriminatory zone' (12). Serial hCG in a stable, reliable patient: The patient with an ectopic pregnancy has a median rise of 25% in 2 days. Mean rise is slower than that for a viable intrauterine pregnancy in 20% women, and the mean fall is slower than that for spontaneous abortions in 8% women. Thus, hCG alone should not be used to rule out the presence of an ectopic pregnancy. A normal intrauterine pregnancy shows at least a 53% rise in hCG level over 2 days, using two serial estimations. The minimum decline of a spontaneous abortion is 21-35% in 2 days. A rise or fall of slower than this is predictive of an ectopic pregnancy (9). Over further follow up monitoring, once hCG rises above 2,500 mIU/mL an ultrasound examination can confirm the presence of an intra-uterine pregnancy. Culdocentesis: It is highly predictive, though it is rarely used since the advent of trans-vaginal ultrasound examinations. Hemoperitoneum (>5 ml of non-clotting blood) in combination with a positive pregnancy test is 99% predictive of a ruptured ectopic. Serum progesterone: These levels are not very helpful when used independently. Values between 10- 20 ng/dL are associated with high risk of ectopic pregnancy (52%), and need to be used in association with hCG and ultrasound especially in patients at high risk of ectopic pregnancy (15). In patients undergoing infertility treatment (ovulation induction), there are higher serum progesterone levels in intrauterine pregnancy and ectopic pregnancy. Values = 25 ng/dL is associated with normal intrauterine pregnancy in more than 97% cases, while values =5 ng/dL is highly predictive of spontaneous abortions. Human Chorionic Gonadotropin (hCG) level above 2500 mIU/mL: An ultrasound examination should be done to help formulate a diagnosis in such patients as the diagnostic efficacy of ultrasound is almost 100% in such patients. However, in case of uncertainty, dilatation and curettage (D&C) is diagnostic. Ectopic pregnancy is established when histo-pathological examination of curetted endometrial lining shows no products of conception (chorionic villi), although as high as 20% women with an intrauterine pregnancy may not have chorionic villi in a curette sample. This procedure unfortunately also leads to the interruption of an intra-uterine pregnancy at the same time. Further, a rising hCG level 24 hours after the D&C indicates the presence of trophoblastic tissue outside the uterine cavity -- reliably suggesting an ectopic pregnancy. In cases where ectopic pregnancy is strongly suspected and the histo-pathological examination is non-diagnostic, a frozen section biopsy may help clarify the diagnosis. Ectopic pregnancy should be strongly considered as a diagnosis in all cases where an intra-uterine sac is not visible on trans-abdominal ultrasound and the serum hCG is above 2,500 mIU/mL. Implantation bleeding of early pregnancy; disturbed intrauterine pregnancy; spontaneous abortion; ruptured functional ovarian cyst; appendicitis; pelvic inflammatory disease; and other gynecological and abdominal conditions that cause abdominal pain are the conditions that can mimic ectopic pregnancy. The aims of treatment are: To treat and limit the progress of the ectopic pregnancy and prevent further complications; to preserve the fertility of the patient. For ampullary ectopic pregnancy the risk of rupture is thought to be approximately 10% when the hCG value is less than 1,000 mIU/mL. For isthmic ectopic pregnancy the risk for rupture is approximately 10% when the hCG value is less than 100 mIU/mL as the space in which isthmic pregnancies must grow is far smaller. Therefore, treatment options must factor in the site of the ectopic. Three primary treatment modalities are available for an ectopic pregnancy: It primarily involves the use of methotrexate and has recently gained popularity as a treatment protocol for avoiding surgical risk. Currently, success rates of methotrexate are defined in terms of resolution of ectopic pregnancy without need for surgery (67 -- 100%). Methotrexate management may result in destruction of the growing pregnancy but is comparatively slow - often taking 4-6 weeks for complete resolution of the ectopic pregnancy. The risk of rupture is present with methotrexate treatment. Methotrexate (MTX) -- it is an antimetabolite that binds to the catalytic site of dihydrofolate reductase, interrupting the synthesis of purine nucleotides and the amino acids serine and methionine, thus inhibiting DNA synthesis and repair and cell replication (10). It is a non-surgical treatment method used for ectopic pregnancies of size less than 3.5 cm with no fetal heart motion. The overall success for treatment of ectopic pregnancy using systemic methotrexate in observational studies ranges from 71.2% to 94.2% (13). Success depends on the treatment regimen used, gestational age, and hCG level. A systemic review of several observational studies reported a failure rate of 14.3% or higher with single dose methotrexate when pre-treatment hCG levels are higher than 5,000 mIU/mL, compared with a 3.7% failure rate for hCG levels less than 5,000 mIU/mL. If hCG levels are higher than 5,000 mIU/mL, multiple doses may be appropriate. Methotrexate crosses the placenta and is also found to be present in the breast milk of a lactating mother. Under no circumstances should methotrexate be given in a normal pregnancy. It can be considered for those women with a confirmed, or high clinical suspicion of, ectopic pregnancy who are hemodynamically stable with an unruptured mass. A candidate for MTX must be able to comply with follow-up surveillance. Before administering MTX, a woman should have a confirmed normal serum creatinine level, normal liver transaminases, and no bone marrow dysfunction indicated by significant anemia, leucopenia, or thrombocytopenia. Typically, these laboratory tests are repeated 1 week after administering methotrexate to evaluate any possible impact on renal, hepatic, and hematologic function. Post-treatment hCG levels should be monitored until a non-pregnancy level is reached. Treatment protocol for using methotrexate to treat ectopic pregnancy: Blood test for a pre-treatment hCG titer, blood group type and Rh, CBC and chemistry profile (with liver enzymes and renal function tests); ultrasound does not show any signs of intrauterine pregnancy; dilation and curettage (D&C) is negative for chorionic villi (if indicated). Get informed consent after discussion about the risks and benefits of treatment, and the definitive treatment of the pregnancy will be agreed to if methotrexate fails. Rhogam if Rh negative, and greater than 7-8 weeks gestation (mini-Rhogam is usually adequate). Patient is instructed to avoid alcohol use, folic acid containing vitamins and sexual relations during treatment. Review the medications that may interact and disallow their use. Non-tubal ectopic pregnancies too are often managed with methotrexate. Cervical, abdominal and cornual pregnancies are very dangerous and require careful consideration of existing treatment options. Severe bleeding can be associated with methotrexate or surgical treatments and very close observation is absolutely necessary until the pregnancy is resolved. Single-dose regimen: Single dose MTX 50 mg/ m2 (body surface area) intramuscular (IM) day 1. Measure hCG level on post-treatment days 4 and 7. Check for 15% hCG decrease between days 4 and 7. Then measure hCG weekly until reaching the non-pregnant level. If results are less than the expected 15% decrease, re-administer MTX 50 mg/m2 and repeat hCG measurement on days 4 and 7 after second dose. This can be repeated as necessary. If, during follow-up, hCG levels plateau or increase, consider repeating MTX. Two-dose regimen: Administer 50 mg/m2 intramuscular (IM) on day 0. Repeat 50 mg/m2 IM on day 4. Measure hCG levels on days 4 and 7, and expect a 15% decrease between days 4 and 7. If the decrease is greater than 15%, measure hCG levels weekly until reaching non-pregnant level. If less than a 15% decrease in hCG levels, re-administer MTX 50 mg/m2 calendar days 7 and 11, measuring hCG levels. If hCG levels decrease 15% between days 7 and 11, continue to monitor weekly until non-pregnant hCG levels are reached. If the decrease is less than 15% between days 7 and 11, consider surgical treatment. Fixed multidose regimen: Administer MTX 1 mg/kg intramuscular (IM) on days 1, 3, 5, 7, alternate daily with folinic acid 0.1 mg/kg IM on days 2, 4, 6, 8. Measure hCG levels on MTX dose days and continue until hCG has decreased by 15% from its previous measurement. The hCG level may increase initially above pre-treatment value, but after 15% decrease, monitor hCG levels weekly until reaching the non-pregnant level. If the hCG level plateaus or increases, consider repeating MTX using the regimen described. Predictor of success: The most commonly identified predictors of successful MTX treatment of ectopic pregnancy include initial serum hCG level, progesterone level, size and volume of gestational mass, presence or absence of cardiac activity, and presence or absence of free peritoneal blood. Of these, hCG levels are most predictive. A recent review of 350 women treated with single-dose MTX for ectopic pregnancy found that the only factor that contributed significantly to the failure rate was serum hCG level before treatment (12). Above 5,000 mIU/mL, the failure rate rose to about 13%. However, there is no absolute level at which medical management is contraindicated. We currently use the multidose protocol or the new "two-dose protocol" to treat women with a hCG above 1,000 mIU/mL. Signs of treatment failure and/or tubal rupture: It is not necessary to follow patient with serial ultrasound examinations once they have received MTX treatment because ultrasound findings would not alter management unless a new tubal rupture is seen. Signs of treatment failure risking possible tubal rupture are -- significantly worsening abdominal pain, regardless of change in hCG levels; hemodynamic instability. Levels of hCG that do not decline by at least 15% between day 4 and day 7 post-injection. Increasing or plateauing hCG levels after the first week of treatment. Failure of the hCG level to decrease by at least 15% from day 4 to day 7 after methotrexate administration is considered treatment failure (14). Therapy with either additional methotrexate administration or surgical intervention is required. Absolute contraindications: Breastfeeding; overt or laboratory evidence of immunodeficiency; alcoholism; alcoholic liver disease or other chronic liver disease; pre-existing blood dyscrasias (i.e. bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia); known sensitivity to methotrexate; active pulmonary disease; peptic ulcer disease; hepatic, renal or hematologic dysfunction. Relative contraindications: Gestational sac larger than 3.5 cm; embryonic cardiac motion. Methotrexate morbidity usually is dose and treatment duration related. Because methotrexate affects rapidly dividing tissues, gastrointestinal side effects, such as nausea, vomiting, and stomatitis, are the most common. Women treated with MTX should be advised not to use alcohol, non-steroidal anti-inflammatory drugs (NSAIDs), folic acid supplements, avoid sunlight exposure and to refrain from sexual intercourse or vigorous physical exercise. Elevation of liver enzymes usually is seen only with multidose regimens and resolves after discontinuing MTX use or increasing the rescue dose of folinic acid. Alopecia is rare side effect and with the doses used to treat ectopic pregnancy. Destruction of bone marrow blood precursors puts patient at risk of developing thrombocytopenia, reticulocytopenia, lymphopenia, and granulocytopenia. Thrombocytopenia predisposes patients to life-threatening hemorrhage and lymphopenia and granulocytopenia that predispose patients to systemic infections (3). There is also potential for nephrotoxicity, interstitial pneumonitis, and an anaphylactic reaction. It is not unusual for women treated with methotrexate to experience abdominal pain 2-3 days after administration, presumably from the cytotoxic effect of the drug on the trophoblast tissue, causing tubal abortion. In the absence of signs and symptoms of overt tubal rupture and significant hemoperitoneum, this pain usually can be managed expectantly by monitoring a woman's hemoglobin level and intraperitoneal fluid amount with transvaginal ultrasonography. Several physicians refer to this as "methotrexate pain" but rupture of the existing ectopic pregnancy must be considered and ruled out. Surgery is the method of choice in all patients who are hemodynamically unstable, ruptured or in whom diagnosis is not possible even after prolonged diagnostic protocols. Surgery promotes the early resolution of symptoms, and drastically eliminates the risk of rupture. It is the method of choice in these patients as it treats the ectopic rapidly and definitively, and does not prolong the growth of the ectopic or risk rupture while diagnosis is pending or while the patient is undergoing medical treatment (methotrexate). However, it does involve surgical and anesthesia risks. Salpingectomy in a hemodynamically unstable patient and in patients with ruptured ectopic pregnancy prevents further complications, stops the further loss of blood and treats the ectopic pregnancy. This is often performed laparoscopically since this involves fewer complications. Salpingostomy in a hemodynamically stable patient in whom definitive diagnosis could not be established or there are contra-indications to medical treatment. This allows for preservation of fertility, reduced post operative recovery period and reduced post operative pain. It also involves a smaller incision and better healing with very little increase in risk of recurrent ectopic. The salpingostomy may be done by either a cautery of a laser. Such patients need to be followed up till serial hCG measurements show a decline of at least 20% over 72 hours or till the value of hCG falls to =5 mIU/mL. Salpingectomy by laparotomy is the traditional and gold standard method of treatment when rupture is imminent and when bleeding is profuse (10). Choice between laparoscopic and open surgery may depend on: clinical spectrum of the disease, prior history of disease in the tubes, desire for future pregnancy, laparoscopic proficiency of the surgeon and the equipment present in the operation room. All laparoscopic patients need to be counseled on the possibility of laparotomy if laparoscopic treatment fails or when an exploratory laparoscopy is being done. Type of surgery may also be based on the following factors: Salpingostomy Versus Salpingectomy: Removal of ectopic pregnancy can be accomplished by resection of the involved fallopian tube with the implanted trophoblastic tissue (salpingectomy) or by dissection and removal of only the ectopic pregnancy with tubal conservation (salpingostomy). Segmental resection of the involved tube with subsequent reanastomosis and tubal reconstruction has fallen out of favor because of the success of bypassing the fallopian tube altogether by using in-vitro fertilization (IVF) (3). The concern with conservative treatment via salpingostomy is that of the persistence of trophoblast tissue due to incomplete removal from the fallopian tube. This problem has been reported as complicating about 5-20% of cases treated with tubal conservation. It is therefore very important to document a complete resolution of the ectopic pregnancy by monitoring hCG values until they return to zero. Levels that fail to drop, or ones that plateau, indicate a likely persistent ectopic pregnancy that should be treated. This can be accomplished successfully in almost all cases where tubal rupture is not present by administration of MTX, with a single dose usually being sufficient. The decision to perform a salpingostomy as opposed to a salpingectomy is often made intraoperatively. In cases of tubal rupture of obvious severe damage, tubal conservation is not indicated. Likewise, if tubal bleeding is encountered that requires extensive coagulation to achieve hemostasis, then future tubal function would likely be compromised, and salpingectomy may be the appropriate intervention. Recurrent ectopic pregnancy in a previously incised tube should also be treated with salpingectomy. It should be actively discouraged owing to the risk of rupture in all tubal pregnancies. Distinguishing patients, who are experiencing spontaneous resolution of their ectopic pregnancies from patients who have proliferating ectopic pregnancies and require active intervention, is difficult. Approximately 20 -- 30% of ectopic pregnancies are associated with declining hCG levels at the time of presentation. When the initial serum hCG is less than 200 mIU/mL, 88% of the pregnancies may have spontaneous resolution. Expectant management is essentially observation and monitoring without active treatment, with the understanding that up to 25% of ectopic pregnancies will resolve on their own. Risk of rupture is present with expectant treatment. If ectopic or spontaneous abortion is confirmed, Rh prophylaxis should be given to Rh-negative women. The treatment options must factor in the site of the ectopic. Criteria that are occasionally used in deciding on expectant management are: decreasing hCG serum values on serial determination; not ruptured, and not associated with serious bleeding or hemorrhage; size of ectopic pregnancy is less than 4 cm; patient strongly resists both surgery and medical management. Reasons for abandoning expectant management include intractable or significantly increased pain, failure of hCG levels to decrease, and tubal rupture with hemoperitoneum (15). Candidates for successful expectant management must be willing to accept the potential risks of tubal rupture and hemorrhage; they should be asymptomatic and have objective evidence of resolution (generally manifested by decreasing hCG levels). Because ectopic pregnancy occurs mostly as a result of fallopian tube pathology, there is substantial risk of recurrence, both at a previously operated tubal site and in the contralateral tube. Thus, women who have undergone salpingectomy still have an increased risk of developing an ectopic pregnancy in the remaining tube. The risk of recurrent ectopic pregnancy after methotrexate treatment is similar to that after salpingostomy, about 10%. Tubal patency after methotrexate treatment is best assessed with hysterosalpingography (HSG), and this has been compared with patency after conservative surgery. Reproductive outcome after a previously treated ectopic pregnancy appears to be similar, whether the treatment method had been methotrexate or conservative surgery (10,12,13). Intrauterine pregnancy rates seem to be comparable in both of these groups, with a possible slightly lower risk of recurrent ectopic seen in the medically treated group. Women who have had a previous ectopic pregnancy should be followed closely during their subsequent pregnancy to ensure its proper site of implantation. However, even among a population of women at increased risk for ectopic, screening them with transvaginal ultrasonography and performing hCG testing when they are asymptomatic does not appear to have much benefit in decreasing morbidity. It is difficult to diagnose an ectopic pregnancy based solely on history and physical examination findings in a community setting. Ectopic pregnancy must be ruled out in every sexually active woman presenting with abdominal pain and bleeding combined with a positive pregnancy test. An early diagnosis is facilitated by the use of sensitive hormonal tests (human chorionic gonadotropin), ultrasound exam, diagnostic laparoscopy, and dilation and curettage of the endometrium. Modern surgical and medical treatments frequently help preserve the involved fallopian tube and avoid extensive surgery. Methotrexate is a viable option with early confirmed diagnosis and a clinically stable condition. Intramuscular methotrexate is an appropriate method for treating selected patients with small, unruptured tubal pregnancies. Successful treatment with methotrexate may require more than one dose of methotrexate. Failure of hCG levels to decrease by at least 15% from day 4 to day 7 after methotrexate administration indicates the need for an additional dose of methotrexate or surgery. There may be a role for expectant management of hemodynamically stable patients with presumptive ectopic pregnancy in whom hCG levels are low (<200 mIU/mL) and declining. Prior studies have shown that over half of women who experience an ectopic pregnancy will have a healthy baby in the future either naturally or with the aid of assisted reproductive technology, although the risk of having another ectopic pregnancy is increased. |