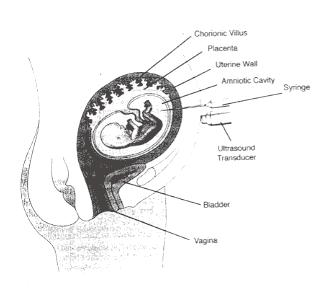
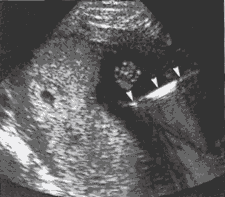
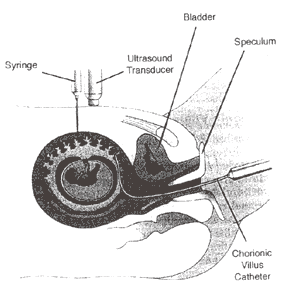
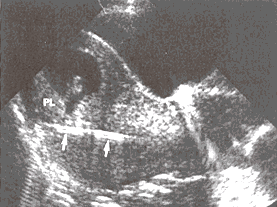
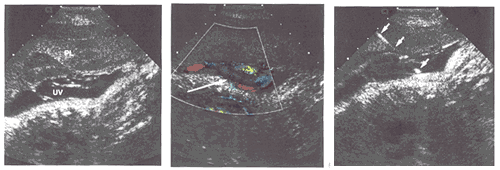
Ultrasound-guided Diagnostic Obstetrical ProceduresWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). Ultrasound emerged as a major tool in medical imaging in the 1970s, and its impact has been very dramatic in obstetrics. The ability of sonography to detect fetal abnormalities prior to delivery and to direct minimally invasive therapy has revolutionized the field of obstetrics. The marked improvement in ultrasound image quality in recent years and the ability to store high quality digital images and video clips have enhanced ultrasound's role in obstetrics. The purpose of this document is to discuss various diagnostic procedures available and their indications. The most commonly used ultrasound-guided diagnostic obstetrical procedures are: Amniocentesis Amniocentesis is aspiration of amniotic fluid through a percutaneously inserted needle. Although amniocentesis can be performed without ultrasound guidance; the use of ultrasound to select a site, guide the needle insertion and monitor the procedure is advisable. Before the needle is inserted ultrasound is used to select a site that permits safe access to the fluid, avoiding the fetus, umbilical cord, large uterine blood vessels and placenta. Continuous real-time monitoring is used throughout the procedure in case fetal movement or uterine contraction requires the needle position to be changed. If needle traverses the placenta, blood is often seen streaming from the placenta into amniotic fluid as soon as the needle is removed. This placental bleeding usually stops within a short time and carries no untoward side-effect. Indications: This procedure is most commonly performed to assess the risk of open neural defect (testing for levels of alpha-fetoprotein or acetylcholinesterase), Down syndrome (fetal karyotype) in the second trimester. Checking for fetal lung maturity is most common indication in the third trimester. Checking for fetal hemoglobin breakdown products in cases of suspected hemolysis due to maternal antibodies to fetal blood is another reason amniocentesis is done. Less frequently amniocentesis is performed therapeutically to reduce the amniotic fluid volume in the cases of polyhydramnios or to treat twin-to-twin transfusion syndrome by taking the fluid from the recipient twin's sac. Risks: amniotic fluid leak, chorioamnionitis, and unexplained post-procedure fetal demise. The pregnancy loss rate after second-trimester amniocentesis has been estimated to be approximately 0.4% (1). Figure 1: Schematic of Amniocentesis Figure 2: Chorionic Villus Sampling (CVS) The chorionic villi develop from the fertilized egg hence; these cells have the same genetic makeup as the fetus. Sampling and testing the villi, via direct examination of the mitotically active cytotrophoblasts and culture of the mesenchymal cells provide chromosomal and biochemical information about the fetus. CVS is usually performed at 10-12 weeks of gestation and karyotypic results are available within 1-7 days. Indication: CVS yields chromosomal information earlier in the pregnancy and more quickly than amniocentesis and may be helpful if the decision of termination of pregnancy is considered because of Down syndrome or malformations. Risks: Pregnancy Loss is higher with CVS than with amniocentesis. The risk may be difficult to compare because CVS is performed earlier in pregnancy and the risk of spontaneous miscarriage is higher in first trimester than in second trimester. Inaccurate karyotype is possible because the placenta and fetus can occasionally have different karyotypes. Contamination of the sample by maternal decidual cells is another potential source of error. Fetal Malformations - an increased incidence of limb reduction anomalies after CVS has been reported. This risk appears to be restricted to CVS performed before 10 weeks of pregnancy (2). Procedure: It is performed under continuous ultrasound guidance and the procedure can be performed via one of two approaches; transabdominal or transcervical. Figure 3: Transabdominal Chorionic Villus sampling, a Transcervical Route: a catheter is inserted through the cervix Figure 4: Schematic of Chorionic Villus Sampling; either Figure 5: Transcervical CVS sampling; a catheter (arrows) Percutaneous Umbilical Blood Sampling It is also termed cordocentesis and is an ultrasound-guided procedure Indications: it is performed for various diagnostic Risks: it carries higher risks of fetal demise or Procedure: if placenta is anterior, the needle is Figure 6: Percutaneous Umbilical Blood Sampling; A - Diagnosing Fetal Aneuploidy: Traditional genetic amniocentesis usually is offered between 15 and 20 weeks of gestation. The fetal loss rate is approximately 0.5%, and minor complications occur infrequently. These include transient vaginal spotting or amniotic fluid leakage in approximately 1-2% of all cases and chorioamnionitis in less than one in 1,000 cases. Needle injuries to the fetus have been reported but are very rare when amniocentesis is performed under continuous ultrasound guidance. Amniotic fluid cell culture failure is uncommon. Safe performance of genetic amniocentesis requires specialized training and ongoing experience. Early amniocentesis, performed from 11 weeks to 13 weeks of gestation, has been widely studied, and the technique is similar to traditional amniocentesis (5). However, early amniocentesis results in significantly higher pregnancy loss and complication rates than traditional amniocentesis 2.5%, compared with 0.7% with traditional amniocentesis. Membrane rupture and amniotic fluid culture failures have higher incidences with early procedure, necessitating an additional invasive procedure for diagnosis. For these reasons, many centers no longer offer early amniocentesis. Indications for chorionic villus sampling (CVS) are similar to those for amniocentesis, except for a few rare genetic conditions that require chorionic villi for diagnosis. CVS is generally performed at 10-12 weeks of gestation. The primary advantage of CVS over amniocentesis is that results are available much earlier in pregnancy, which provides reassurance for parents when results are normal, and when results are abnormal, allows earlier and safer methods of pregnancy termination. Skill in ultrasound-guided procedures and extensive specialized training are required before attempting CVS, and maintenance of skills with regularly scheduled procedures is essential. Relative contraindications to CVS include vaginal infections (including chlamydia and herpes), vaginal bleeding or spotting, extreme anteversion or retroversion of the uterus, and patient body habitus precluding easy access to the uterus or clear visualization of intrauterine structures with ultrasonography. Several major collaborative trials report success rates of more than 99% with cytogenetic analysis and total pregnancy loss rates of 0.6-0.8% for CVS in excess of traditional amniocentesis (6). Patients considering CVS should be counseled that there may be a slightly higher risk of pregnancy loss associated with CVS than with traditional amniocentesis. In an analysis by the World Health Organization, an incidence of limb reduction defects of 6 per 10,000 was reported, which is not significantly different from the incidence in the general population. Women considering CVS who are concerned about the possible association of CVS with limb defects can be reassured that when the procedure is performed after 9 menstrual weeks, the risk is low and probably not higher than the general population at risk. Although the risks of pregnancy loss are relatively low, lack of adequate controls tends to underestimate the true added risk of prenatal invasive procedures (7). Cordocentesis, also known as percutaneous umbilical blood sampling, involves puncturing the umbilical vein under direct ultrasound guidance. Karyotype analysis of fetal blood usually can be accomplished within 24-48 hours. The procedure-related pregnancy loss rate, including all indications for the procedure, has been reported to be less than 2% (8). References: Editor's Note Developed from one of the best and broadest sonographic collection, |