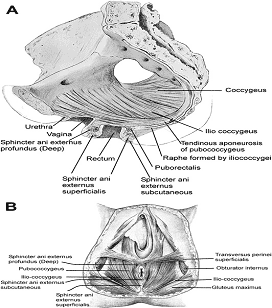
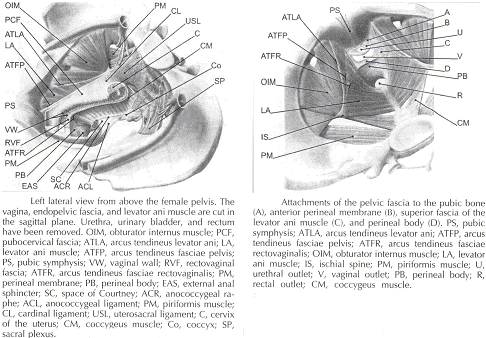
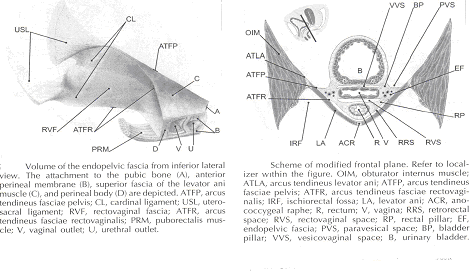
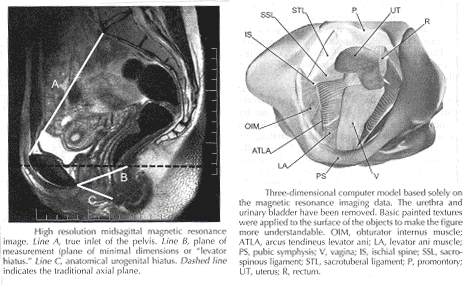
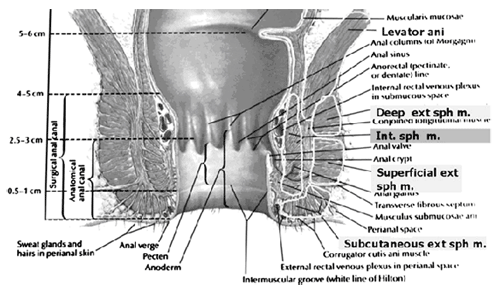
Current Concepts in Pelvic Floor AnatomyWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). To understand incontinence, it is important to understand the pelvic anatomy. The lower urinary tract consists of the bladder and urethra in women and the bladder, urethra, and prostate in men. These structures are intimately supported by the muscles and ligaments of the pelvis, and innervated by the cortex, brain stem, and thoracolumbar and sacral segments of the spinal cord. The bony pelvis is composed of the pubic bone, the ischial bone, and the iliac bone. The bottom of this bony pelvis is lined by a broad sheet of muscle, the pelvic diaphragm. Three openings in this diaphragm accommodate the urethra, vagina (in women), and rectum. The side of pelvic muscles that faces the abdominal contents is covered by endopelvic fascia. The endopelvic fascia is a confluent suspensory apparatus of the female pelvic organs. Pelvic floor muscles and specialized connective tissues provide support or act as a floor for the abdominal viscera including the rectum; and they provide constrictor or continence mechanism to the urethral, anal, and vaginal orifices (in females). Failure of the pelvic diaphragm and the connective tissues results in incontinence and pelvic prolapse. Childbirth is considered a significant risk factor for pelvic floor dysfunction. The study of the pelvic floor is difficult for several reasons. First, this region is often inaccessible because it is enclosed by the pelvic bones. This relatively small space also contains many organ systems, and selected structures are observable only by special dissections made by sacrificing other structures. In addition, during surgery or cadaver dissection, the strength differs from the normal state because of the altered tone of the muscles. Knowledge of the embryology of the genitourinary system is necessary for understanding the causes of the multiple congenital anomalies of the upper and lower urinary tracts. The purpose of this document is to explore current concepts in pelvic floor anatomy and support. The endopelvic fascia divides the lesser pelvis in a manner that is similar to the way the urorectal septum divides the embryonic cloaca. Connecting descriptions of the geometry of the organs visible by magnetic resonance imaging with descriptions of their individual connections to the endopelvic fascia is also discussed. The study aims to discuss the applied anatomy and embryology of pelvic floor structures. The relevance of pelvic floor to anal opening and closure function discusses new findings with regards to the role of three muscles in the vaginal closure mechanisms. The anal-rectal angle was previously thought to be important in maintaining fecal continence, but its importance has been questioned. More recent studies suggest that fecal incontinence in women is often related to denervation of the muscles of the pelvic diaphragm, as well as to disruption and denervation of the external anal sphincter. EmbryologyLower Urinary Tract: At approximately 15 days after fertilization, invagination and lateral migration of mesodermal cells occur between the ectodermal and endodermal layers of the presomite embryo. These migrating cells form the intraembryonic mesoderm or mesodermal germ layer. By the 17th day of development, the endoderm and ectoderm layers are separated entirely by the mesoderm layer, with the exception of the prochordal plate cephalically and the cloacal plate caudally. The cloacal plate consists of tightly adherent endodermal and ectodermal layers. Concomitantly, at about the 16th day of development, the posterior wall of the yolk sac forms a small diverticulum, the allantois, which extends into the connective stalk. With ventral bending of the embryo cranially and caudally during somite development, the connecting stalk and contained allantois, as well as the cloacal membrane, are displaced onto the ventral aspect of the embryo. The hindgut undergoes slight dilatation to form the cloaca; it receives the allantois ventrally and two mesonephric ducts laterally. Ventral mesodermal elevations occur, forming the urethral folds (primordial of the labia minora) and gential tubercle (primordium of the clitoris). A spur of mesodermal tissue migrates from the base of the allantois toward the cloacal membrane around 28 days after fertilization, forming the urorectal septum. This structure partitions the cloaca into a ventral urogenital sinus and a dorsal rectum. The urogenital opening (future vestibule) is formed by the independent involution of the urogenital membrane. The point at which the urorectal septum intersects with the cloacal membrane will become the perineal body (1). By 28 days of development, the mesonephric ducts have reached and fused with the urogenital sinus. At this time, the ureteric bud appears as a diverticulum from the posteromedial aspect of the mesonephric duct at the point where the terminus of the duct bends to enter cloaca. The ureteric bud branches and dilates to create the renal pelvis, major and minor calyces, and collecting ducts. The renal blastema originates at the level of the upper sacral segments. The final position of the kidney at the level of the upper lumbar vertebrae is attributed to ascent of the renal blastema. Formation of bladder, trigone, and urethra: At the point of its connection with the mesonephric ducts, the urogenital sinus is divided into the vesicourethral canal cranially and the definitive urogenital sinus caudally. Dilation of the cranial portion of the vesicourethral canal forms the definitive bladder, which is of endodermal origin. The vesicourethral canal communicates at its cranial end with the allantois, which becomes obliterated at about 12 weeks of fetal life, forming the urachus. This structure runs from the bladder dome to the umbilicus and is called the median umbilical ligament in the adult. The ureteric bud begins as an outgrowth of the mesonephric duct, but with positional changes of the embryo during growth, the mesonephric duct and the ureteric bud shift positions so that the ureter comes to lie posterolaterally to the duct. The segment of the mesonephric duct distal to the site of origin of the ureteric bud dilates and is absorbed into the urogenital sinus, forming the bladder trigone. This structure effectively gives the endodermal wall of the vesicourethral canal a mesodermal contribution. At about 42 days after fertilization, the trigone may be defined as the region of the vesicourethral canal lying between the ureteric orifices and the termination of the mesonephric ducts. The caudal portion of the vesicourethral canal remains narrow and forms the entire urethra. The separate development of the trigone and bladder may explain why the muscle laminae of the trigone are contiguous with the muscle of the ureter, but not with the detrusor muscle of the bladder. This separate development also may account for pharmacologic responses of the musculature of the bladder neck and trigone, which differ partially from those of the detrusor. Time-table of events in the development of the lower urinary tract -- Time after fertilization (1): 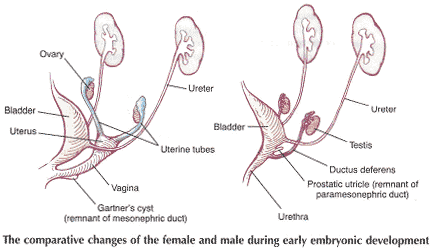 Rectum and Anal Sphincters: The hindgut, which in embryo extends from the posterior intestinal portal to the cloacal membrane, gives rise to the distal third of the transverse colon, the descending colon, the sigmoid, the rectum, and the upper part of the anal canal. The terminal portion of the hindgut enters into the cloaca, and endoderm-lined cavity that is in direct contact with the surface ectoderm. In the contact area between the endoderm and ectoderm, the cloacal membrane is formed. During further development a transverse ridge, the urorectal septum, arises in the angle between the allantois and the hindgut. This septum gradually grows caudad, thereby dividing the cloaca into an anterior portion, the primitive urogenital sinus, and a posterior portion, the anorectal canal (2). The primitive perineum is formed when the urorectal septum reaches the cloacal membrane when the embryo is 7 weeks old. The cloacal membrane is thus divided into the anal membrane posteriorly and the urogenital membrane anteriorly. The anal membrane is then surrounded by mesenchymal swelling and in the ninth week it is found at the bottom of an ectodermal depression, known as the anal pit. The surrounding swellings are the anal folds. Soon thereafter the anal membrane ruptures and an open pathway is formed between the rectum and the outside, which at this stage of development is the amniotic cavity. The upper part of the anal canal is thus endodermal in origin; the lower third of the anal canal is ectodermal. The external anal sphincter appears in human embryos at approximately 8 weeks or perhaps 7 weeks. This sphincter, together with the levator ani, is believed to originate from hypaxial myotomes. Although the anal sphincter and levator ani may arise from distinct primordial, their relationship is very close. 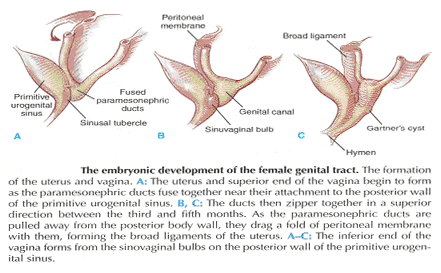 Schematic representation of the embryologic contributions of various structures of the female urogenital system: Pelvic Organ SupportPelvic Floor: The pelvic floor is made up of muscular and fascial structures that enclose the abdominal-pelvic cavity, the external vaginal opening (for intercourse and parturition), and the urethra and rectum (for elimination). The fascial components consist of two types of fascia: parietal and visceral (endopelvic). Parietal fascia covers the pelvic skeletal muscles and provides attachments to muscles to the bony pelvis; it is characterized histologically by regular arrangements of collagen. Visceral fascia is less discrete and exists throughout the pelvis as a meshwork of loosely arranged collagen, elastin, and adipose tissue through which the blood vessels, lymphatics, and nerves travel to reach the pelvic organs. By surgical convention, the visceral endopelvic fascia of the pelvis has been described as discrete "ligaments" such as the cardinal or uterosacral ligaments. The pelvic floor is comprised of a number of muscles and they are organized into superficial and deep muscle layers. There is significant controversy with regards to the nomenclature, but generally speaking the superficial muscle layer and the muscles relevant to the anal canal function are the external anal sphincter (EAS), perineal body, and possibly the puboperineal (or transverse perineal) muscles. The deep pelvic floor muscles consist of pubococcygeus, ileococcygeus, coccygeus, and puborectalis muscles. Puborectalis muscle is located in between the superficial and deep muscle layers, and it is better to view this as the middle muscle layer of the pelvic floor. In addition to the skeletal muscles from the rectum, caudal extension of the circular and longitudinal smooth muscles from the rectum into the anal canal constitutes the internal anal sphincter (IAS) and EAS of the anal canal, respectively (3). Pelvic Diaphragm: The pelvic diaphragm collectively consists of the levator ani muscle and associated connective tissue attachments to the pelvis. The diaphragm is stretched hammock-like between the pubis in front and the coccyx behind, and is attached along the lateral pelvic walls to a thickened band in the obturator fascia, the arcus tendineus levator ani. The levator ani functions as a unit but is described in two main parts: the diaphragmatic part (coccyx and ileococcygeus muscles) and the pubovisceral part (pubococcygeus and puborectalis). The coccygeus muscles are paired and run from the lateral borders of the coccyx and sacrum to the ischial spines on each side, with the sacrospinous ligaments forming the tendineus components. The ileococcygeus muscle arises from the lateral pubic symphysis; travels over the pelvic sidewall (obturator internus muscle) attached to the arcus tendineus components. The ileococcygeus muscle arises from the lateral pubic symphysis, travels over the pelvic sidewall (obturator internus muscle) attached to the arcus tendineus levator ani laterally, and meets in the midline at the anococcygeal raphe and the coccyx to form the levator plate. The pubovisceral portion of the levator ani arises from the inner surface of the pubic bones and passes backward to insert into the anococcygeal raphe and the superior surface of coccyx. The levator crura are formed by the pubococcygeus and puborectalis muscles, which have attachments to the lateral aspects of the vagina and rectum. The puborectalis forms a sling behind the rectum and contributes to anal continence. The space between the levator crura through which rectum, vagina and urethra pass is called the genital hiatus (4). The levator ani exhibits constant baseline tone and can also be voluntarily contracted. The muscle contains both type I (slow-twitch) fibers to maintain constant tone and type II (fast-twitch) fibers to provide reflex and voluntary contractions. Innervation is provided primarily through the anterior sacral nerve roots of S2, S3, and S4; additional innervation may be provided to the pubovisceral components through branches of the pudendal nerve, although this is controversial. Perineal Membrane: The perineal membrane is a triangular sheet of dense fibromuscular tissue that spans the anterior half of the pelvic outlet. It had previously been called the urogenital diaphragm and according to DeLancey (1988), this change in name reflects the appreciation that it is not a two-layered structure with muscle in between, as had been thought. The vaginal and urethra pass through the perineal membrane and are supported by it. Cephalad to the perineal membrane lies the striated urogenital sphincter muscle, which, as already mentioned, compresses the distal urethra (5). The lower vagina is supported predominantly by connections to the perineal membrane anteriorly and the perineal body posteriorly. Endopelvic Fascia in Women: The continuity of the various parts of the suspensory apparatus of the pelvic floor organs has been well known for more than 150 years. However, this reality is often not emphasized enough. The female pelvic organs are connected to the pelvic sidewall by a network of connective tissue strands commonly called the endopelvic fascia. This fascia forms one continuous unit -- composed of collagen, elastin, and non-vascular smooth muscle fibers and is penetrated by blood and lymph vessels and nerves. Some parts of endopelvic fascia have traditional names that often overlap, leading to confusion. The endopelvic fascia has the shape of a semifrontally oriented septum, which surrounds the vagina and part of the uterine cervix and divides the pelvic floor into the anterior and posterior compartments. This confluent septum has specific connections to the pubic bone, anterior perineal membrane, perineal body and superficial fascia of the levator ani muscle. Additionally, the uterosacral part of the septum has three subdivisions -- the "vascular part", the "neural part", and the true uterosacral ligament. Each of these subdivisions has a different physical link to the parietal structures. The embryonic origin of the endopelvic fascia remains an interesting question. The endopelvic fascia divides the lesser pelvis in much the same fashion as the urorectal septum divides the embryonic cloaca. It is possible that the vagina stems from break-up of septum. This would explain why the vagina is surrounded by endopelvic fascia, while the urinary bladder and rectum are not. Thus far, the remnants of the urorectal septum have been considered to comprise only the perineal body. It seems more probable that the perineal body originates from the complex of the cloacal membrane and the cloacal sphincter, together with the perineal membrane, external urethral sphincter, external anal sphincter, and anococcygeal ligament (6). The aspect of the fascia facing the urinary bladder is usually called the pubocervical fascia. It inserts into the pubic bone, except for a few millimeters in the midline, and beneath this insertion it fades into the anterior part of perineal membrane. Laterally, the endopelvic fascia inserts into the superior fascia of the levator ani muscle. The anterosuperior leaf (pubocervical fascia) and inferoposterior leaf (rectovaginal fascia) of endopelvic fascia are connected at both sides of the vagina and craniodorsally fade into the cardinal and further uterosacral ligaments. The vagina is therefore, circularly surrounded by the endopelvic fascia. The adventitia of the base of the urinary bladder is connected to the "pubocervical fascia" by the left and right "bladder pillar". These structures contain terminal branches of middle and inferior vesical artery. The bladder pillars separate the vesicovaginal space from the left and right paravesical space. The vesicovaginal space is closed from below by tight attachment of the middle urethra to the anterior vaginal wall and from above by the supracervical septum (7). The rectum is wrapped in its fascia ("perirectal fascia" and "fascia propria recti"). This fascia contains branches of the middle rectal artery, veins, lymph vessels, and nerves, which are embedded in a variable amount of fatty tissue. The anterior and posterior leaf of the perirectal insert into the inferoposterior aspect of the endopelvic fascia, close to where the endopelvic fascia attaches to the superior fascia of the levator ani muscle, together constituting the so-called "rectal pillars" or "lateral ligaments of rectum" (8). The rectal pillars separate the rectovaginal space from the retrorectal space. These figures show the spatial relationship of the prevesical, paravesical, vesicovaginal, rectovaginal, and retrorectal spaces to the endopelvic fascia, bladder pillars, and rectal pillars. The aspect of endopelvic fascia facing the rectum is typically called rectovaginal fascia. It fades into the perineal body inferiorly and inserts into the superior fascia of the levator ani laterally. The superior part of the pubocervical fascia fades into the cardinal ligaments, and these further fade into the uterosacral ligaments. The endopelvic fascia does not contain any fatty tissue. Only in the region of the uterosacral ligament do the strands of fibrous tissue run in a fan-like shape in a large area through the subperitoneal adipose tissue. There is a variable amount of fatty tissue outside the adventitia of the urinary bladder and rectum. The density and strong hold of the endopelvic fascia is not uniform in all regions. The tissue density increases in the craniocaudal direction. The tissue is strongest and most dense, and its surface is best defined in the region of the distal pubocervical and distal rectovaginal fascia. In contrast, in the regions of the uterosacral ligament, the strands of fibrous tissue are fine and scattered in the surrounding subperitoneal fat. Furthermore, there is a plexiform crossover of the vessels and nerves of individual organs in this region. In the distal part of the fascia, the vessels and nerves for the vagina, bladder base, and urethra run in a parallel fashion along the edge of the vagina. There is no single method that allows visualization and analysis of all the structures within the female pelvic floor, a three dimensional computer model can be created integrating complimentary information form magnetic resonance imaging (MRI), direct observation from dissection laboratory and from surgical rooms, and the relevant scientific literature. MRI is a useful tool to visualize three-dimensional representation of the anatomy of lesser pelvis and description of the geometry of organs visible with description of their individual connections to the endopelvic fascia (9). Rectum and Anal Sphincters: The rectum extends from its junction with the sigmoid colon to anal orifice. The distribution of smooth muscle is typical for the intestinal tract, with inner circular and outer longitudinal layers of muscle. At the perineal flexure of the rectum, the inner circular layer increases in thickness to form the internal anal sphincter (IAS). The autonomic nerves, sympathetic (spinal nerves) and parasympathetic (pelvic nerves), supply IAS. Sympathetic fibers originate from the lower thoracic ganglia to form the superior hypogastric plexus. Parasympathetic fibers originate from the 2nd, 3rd, and 4th sacral nerves to form the inferior hypogastric plexus, which in turn gives rise to superior, middle, and inferior rectal nerves that ultimately supply the rectum and anal canal. Most of the tone of IAS is myogenic (ie, caused by unique properties of the smooth muscle itself). Angiotensin 2 and prostaglandin F2 α play modulatory roles. Sympathetic nerves mediate IAS contraction through the stimulation of α and relaxation through β1, β2 and β3 adrenergic receptors (10). Stimulation of parasympathetic or pelvic nerves causes IAS relaxation through nitric oxide -- containing neurons located in the enteric plexus. The IAS is responsible for 85% of resting anal pressure. The outer longitudinal layer of smooth muscle wall of the rectum, with connections to the perineal body and coccyx, and then passes inferiorly on both sides of the external anal sphincter. The external anal sphincter (EAS) is composed of striated muscle that is tonically contracted most of the time and can also be voluntarily contracted. Various divisions of the EAS have been described, and although there is no consensus, recent descriptions favor superficial (combining the previous superficial and subcutaneous components) and deep compartments (10). The EAS functions as a unit with puborectalis portion of the levator ani muscle group. The anal sphincter mechanism comprises the internal anal sphincter (IAS), external anal sphincter (EAS) and the puborectalis muscle. As the bladder neck and urethra, a spinal reflex causes the striated sphincter to contract during sudden increases in intraabdominal pressure, such as coughing. The anal-rectal angle is produced by the anterior pull of the puborectalis muscles. These muscles form a sling posteriorly around the anorectal junction. The anal-rectal angle was previously thought to be important in maintaining fecal continence, but its importance has been questioned. More recent studies suggest that fecal incontinence in women is often related to denervation of the muscles of the pelvic diaphragm, as well as to disruption and denervation of the EAS (4). Clinical Applications: Mechanisms of normal support of uterus and vagina: Normal pelvic support is provided by interaction between the pelvic muscles (levator ani group) and connective tissue attachments. Under most conditions, the pelvic muscles are the primary support for the pelvic organs, providing a firm yet elastic base on which they rest. The connective tissue attachments stabilize the pelvic organs in the correct position to receive optimal support from the pelvic muscles. When the pelvic muscles are relaxed, as during micturition or defecation, the connective tissue attachments temporarily hold up the pelvic organs. When the vagina is normally supported, it provides support to the bladder and urethra, cervix, and rectum. Other than the cervix, the uterus does not have fixed supports, as indicated by its ability to enlarge without restriction during pregnancy. The upper two thirds of the vagina is in a nearly horizontal orientation in a standing woman. Connective tissue attachments stabilize the vagina at different levels (4). The superior and lateral connective tissue attachments (cardinal-uterosacral ligament complex, also known as level I) uphold the cervix and upper vagina over the levator plate and away from the genital hiatus. The mid-vagina is supported by lateral connections to the white line or arcus tendineus fasciae pelvis (level II). The lower vagina is supported predominantly by connections to the perineal membrane anteriorly and the perineal body posteriorly (level III). It is important for surgeons working in the paravesical spaces to fully understand the relationship of the most prominent landmark of this region -- the arcus tendineus fasciae pelvis -- to the margin of the levator muscle -- the arcus tendineus levatoris ani. The arcus tendineus levator ani represents the upper margin of the aponeurosis of the ileococcygeus muscle. This aponeurosis is composed of the degenerated upper part of the ileococcygeus muscle and its investing fasciae. During phylogeny of this muscle, there is descent of its origin on the lateral wall of the pelvic cavity form the ilio-pectineal line to the fascia obturatoria. In the dorsal position, the aponeurosis inserts into the ischial spine and into the neighboring anterior margin of the greater sciatic notch. The line of attachment of the aponeurosis of the levator ani may assume any position between the pelvic brim and a horizontal line drawn line drawn through the ischial spine to the back of the body of the pubis (11). This observation, together with many unrecognized obstetric tears, accounts for great variability in the level of insertion seen in dissection and during surgical repair. Childbirth-related injury to the nerves and muscles of the pelvic floor may lead to the development of urinary or fecal incontinence. Pelvic magnetic resonance imaging (MRI) studies have shown that visible puborectalis muscle damage occurs singularly in vaginally parous women and is considerably more prevalent in parous women with stress incontinence than in those without the condition. The studies have shown a maximum stretch ratio of 3.5 to 1 in the posteromedial puborectalis during simulated childbirth that increased with increasing with increasing stiffness of lateral levator attachments (12). Increasing stiffness of the lateral attachments of the levator ani to the pelvic side wall causes increased stretch on the levator ani muscles. Stiffer levator attachments in the nullipara may subject the levator to higher stretch ratios during childbirth, increasing risk for muscle damage. This preliminary work may help explain epidemiologic data regarding the pelvic floor impact of a first delivery. Pelvic floor muscles have two important functions: they provide physical support to the pelvic viscera; and they provide constrictor mechanism to the anal canal, vagina, and urethra. Newer imaging and physiologic studies strongly suggest that these two functions of the pelvic floor are quite distinct and are likely related to different components of the pelvic floor muscles. The pubococcygeus, ileococcygeus, and ischiococcygeus most likely provide the physical support or act as a floor for the pelvic viscera. The puborectalis muscle provides the constrictor function to the anal canal, vagina, and urethra. Levator ani dimensions can be determined by 3D pelvic floor ultrasonography and pelvic MRI. The term "endopelvic fascia" is widely used by clinicians as described above but is not accepted by many anatomists. According to current anatomical nomenclature, the described "endopelvic fascia" is part of the visceral pelvic fascia (fascia pelvis visceralis). The term "endopelvic fascia" is reserved for the parietal pelvic fascia (fascia pelvis parietalis). In this anatomical concept, the visceral pelvic fascia is further subdivided according to the organ that it covers. There is agreement that the composition of the fascia pelvis visceralis is much different from what "fascia" generally means the cover of skeletal muscle. The modeling technique permits further exploration of the relationships between varying maternal-fetal labor parameters (eg, levator ani muscle characteristics, bony pelvic shape, and fetal head geometry) and muscle stretch in labor. The modeling technique permits the inclusion of updated model parameters (eg, muscle/attachment stiffness, tensile strength, and muscle fiber direction) as such parameters become available in the future. Perhaps more sophisticated versions of these models will help to pinpoint specific modifiable aspects of childbirth that may hold the key to preventing childbirth-related pelvic floor injury in the future. Acknowledgment: Special thanks to Dr. Dr. R. K. Mittal, Pelvic Floor Dysfunction and Disorder Group, Division of Gastroenterology, University of California, San Diego VA Health Care Center, San Diego, CA (USA) for the assistance in preparing the manuscript. |