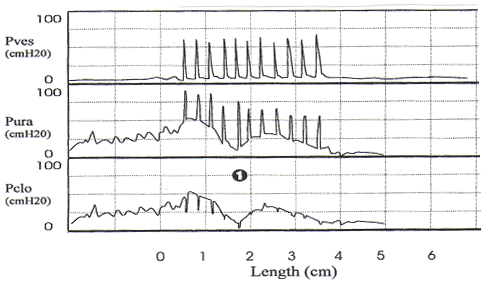
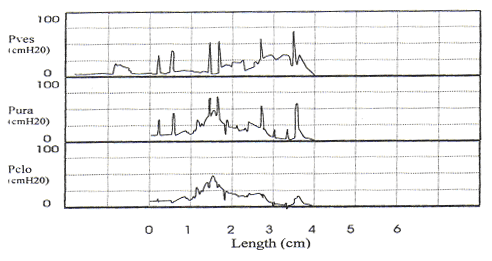
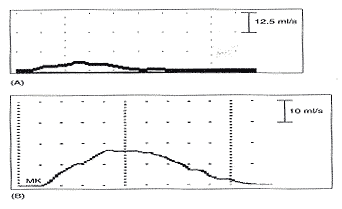
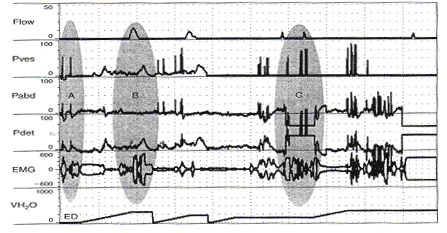
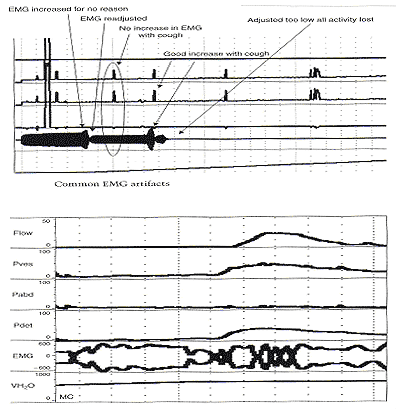
Pitfalls in Urodynamic Studies InterpretationWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). Urodynamic studies provide insight into the functioning of individual components of the lower urinary tract, bladder, and urethra, and into their interactions. Debate continues on the role of urodynamic studies in prediction of surgical outcome and in patient counseling before surgery. Urodynamic evaluation includes a non-invasive flow measurement, filling cystometry and a pressure-flow study. The Urinary Incontinence Treatment Network has provided a multicenter protocol for urodynamic testing and for the interpretation of guidelines and reference values of women with stress incontinence. Urodynamic studies are invaluable in delineating the pathophysiology of lower urinary tract symptoms, but there are many potential sources of error that may cause difficulty in interpretation of data obtained. Sampling error is of two generic kinds, one is physiologic and the other temporal. It is important not to over- or under-interpret the studies. Temporal sampling errors relate to how frequently the data points are recorded and displayed. When the tracing is compressed so that the entire study can fit on a single page, much information is lost. A significant problem in urodynamic evaluation is an inappropriate interpretation of an observation. Often, it is not the measurement that is in error, but rather the interpretation. The validity of a test refers to its ability actually to measure what it is supposed to measure. The validity of the different urodynamic tests is difficult to establish. The clinician involved in urodynamic studies should always be aware of study validity and make every effort to ensure that the studies are as valid as possible. The purpose of this review is to set forth general principles of pitfalls in urodynamic studies and illustrate with a few representative tracings. The refinement of urodynamic techniques, in the context of rapidly evolving strategies to treat stress urinary incontinence, pelvic-organ prolapse and overactive bladder, has allowed physicians caring for women with disorders of the pelvic floor to bladder function more accurately. This review also highlights some of the ongoing debates over the performance, interpretation, and utility of urodynamic testing, and provides references for further reading on these topics. Problems arise if the urodynamic studies are of poor quality or difficult to interpret, and if repeat testing is required. Post-void residual (PVR) urine volume integrates the activity of the bladder and outlet during emptying. It can be measured directly or estimated by ultrasonography. It is useful (and easy) to determine residual volume at least twice during an urodynamic evaluation, because anxiety or discomfort in addition to true pathology may be responsible for an incomplete voiding event. After recording PVR, the patient should be asked whether or not the micturition was normal for him / her, if not, the results should be so recorded and repeated at another time. The major weakness of just relying on PVR is that it does not correlate with intravesical pressure, and more importantly, its poor test -- retest reliability. Despite these scientific flaws, measurement of PVR is taking on more significance as the use of bladder ultrasound for the estimation of bladder volume has become widely commercially available. By comparing the ultrasound measurements with the catheterization urine volume, the sensitivity and specificity of ultrasound detecting the presence of residual urine volumes of >100 ml were excellent. Even though the test -- retest reliability is poor, a series of PVR measurements, when annotated by the clinical circumstances, provides useful information. It is our practice to use catheter to check PVR at time of urodynamic testing when catheterization is required and to use ultrasound to measure PVR in office setting to avoid catheterization. Uroflow and determination of PVR urine are the two most commonly performed urodynamic studies and these are subject to a number of potential errors. First and foremost, it is essential to determine whether or not the recorded uroflow and PVR is representative of the patient's usual voiding. The influence of bladder volume on uroflow is well known and while conventionally a minimum voided volume of 150 mL is thought to be requisite in order to properly assess uroflow, clearly many patients' flow rates continue to increase with voided volumes above that arbitrary cut point. Another artifact may occur depending on the time interval over which data are sampled and displayed. To avoid reporting values of flow that are artifacts due to sudden burst of increased uroflow (usually due to jostling the flowmeter), it is recommended that only flow events lasting 2 seconds or more be reported. A catheter in the urethra may affect flow in two ways, firstly, while the normal urethra usually allows unobstructed voiding over 7 French urodynamic catheter, this is not always the case. In some patients, the catheter itself causes obstruction. It is postulated that in such patients, urethral compliance is abnormal (low) even though the urethra is not narrow enough to cause obstruction. Secondly, the patient may not void in his or her usual fashion because of the presence of the catheter. In addition, one must always remember to take the voided volume into account when comparing the two tracings because uroflow is significantly affected by voided volume. When performing cystometry using an electronic transducer and an electronic recorder, calibration of the equipment needs to be done on a regular basis. The rate of bladder filling during cystometry is an important parameter to monitor and should be measured and recorded with each study. There are some cystometric features such as compliance that can be significantly altered by the rate of bladder filling. A medium-fill rate (10 to 100 mL/min) is commonly used. Select a filling rate that remains constant for a particular laboratory because it is easier to interpret the results over a long period of time. If a gravity-filling technique is used, accurate measurement of the infused volume and the time of infusion should be done, which will allow determination of the infusion rate. Determination of bladder capacity by the cystometrogram may be inaccurate. The rapid filling rate used in carbon dioxide cystometry is not physiologic. It is much too fast. The measured capacity is usually less than the actual capacity because of rapid filling. Patient cooperation is imperative in obtaining adequate cystometric studies. Moving, crying, extraneous muscular activity and failure of the patient to follow directions diminish the value of the study. Recordings of abnormal movement, or coughing should be labeled on the tracings. The operator should note all such visible artifacts so that these pressure spikes will not be interpreted as bladder contractions. Abdominal and vesical pressure should always go up equally during coughing and straining. In the Pves and Pabd transducers are calibrated properly and zeroed to atmosphere, when the stopcock is opened to the patient, both pressures will increase in tandem and detrusor pressure will remain unchanged. The two most common problems with unequal pressure transmission are damping. In order to determine if damping is the problem, carefully examine the tracing spike during a cough. If there are more fluctuations in one of the tracings than the other, there is damping of the channel with diminished fluctuations. The two most common causes of damping are air bubbles in the line and obstruction of the catheter because it is up against the wall of the bladder or rectum. Another source of artifact occurs when, after the initial establishment of satisfactory transducer calibration, one of the catheters moves or is expelled. Recognition of this factor enables the technician to reposition the rectal catheter and avoid losing valuable data during the filling and voiding phases of the study. Yet, another cause of artifact is rectal contractions. Since Pdet is electronically derived by the subtraction of Pabd from Pves, spontaneous rectal contractions cause an artifactual rise in Pdet. Vesical pressure is affected by: 1) the quantity of fluid or gas in the bladder; 2) bladder compliance; 3) rate of filling; and 4) extravesical condition resulting in compression or restriction of expansion of the bladder. Cystometric recordings are affected by any change that alters these factors including vesicoureteral reflux, bladder diverticula and leakage of gas or water from around the catheter. Infections, inflammation and radiation tend to increase the filling limb of the cystometrogram, as do parasympathomimetic drugs. Other medications, such as propantheline, oxybutynin and flavoxate, may decrease bladder tone or increase the volume at which detrusor contractions occur. Cystometric recordings are affected by changes in the position of the patient. In the supine position, patients are less likely to void or leak urine. With patients in the sitting and in the upright positions, baseline bladder pressure and detrusor response to filling are different from those recorded on a patient in the supine position. Other factors that may lead to unusual cystometrograms are abdominal or perivesical masses, patient-anxiety and diuresis. Despite the original presumption that the urethral hypermobility and LPPs were related, it has been demonstrated repeatedly that there is no correlation between two. Further, some published data from others have shown that there are a number of pitfalls with this technique. Firstly, the numeric value of LPP usually decreases with increasing bladder volume and many patients who do not leak at a volume of 150 mL will leak at higher bladder volumes. Secondly, the presence of the catheter, particularly in patients with low urethral compliance may cause a false elevation in LPP. Some patients do not leak at all with the catheter in place and have obvious sphincteric incontinence and a low ALPP once the catheter is removed. In these patients, the catheter size also affects LPP; the larger the catheter, the higher the LPP. Thirdly, radiologic visualization of the leakage is much less sensitive than direct visualization of the urethral meatus. It should be recognized that it is the vesical and not the abdominal pressure which actually provides the energy to drive urine across the sphincter and cause incontinence. Thus, when one is using ALPP clinically it is important to (mentally) add back the estimated detrusor pressure to the ALPP to attain a true estimate of VLPP. The urethral pressure profile (UPP) curve is thus the result of a number of factors, including urethral wall compliance, resistance to inflow of the medium, resistance to runoff of the medium into the bladder and out the urethral meatus, and artifact generated by the apparatus. Because the study is usually done at rest and not during bladder filling or emptying, it is difficult to associate the recorded events with bladder-urethral interaction during filling or emptying. The static UPP has been advocated by some investigators as a useful tool for diagnosing stress incontinence, detrusor sphincter dyssynergia, and obstruction. Generally, it is not useful for the specific diagnosis of any of these. There is much overlap in maximum urethral pressures and functional urethral lengths between individuals with stress incontinence and normal individuals, especially in the elderly population. A high urethral pressure at a given point in the passage of the catheter from the bladder through the urethra can be secondary to a number of phenomena, and this by no means indicates that there will be contraction in this area during voluntary or involuntary micturition. The static infusion urethral profile is useful for testing the function of an artificial genitourinary sphincter as well as diagnosing a non-functional bladder neck or proximal urethral segment. In an individual with such an abnormality, the proximal urethra is isobaric or almost isobaric with the bladder and the striated sphincter peak is generally lower than normal. The status of the bladder may have a significant effect on the UPP. If the bladder capacity is small or if the bladder is hyperreflexic micturition may occur, obscuring the UPP. When performing a stress UPP with a dual-channel microtip transducer catheter, accurate measurement of the differential pressure is dependent on the transducers being calibrated identically. To avoid the pitfall of improper calibration secondary to differences in amplifier gain, stress maneuvers should be performed before the UPP measurement with both the proximal and the distal transducer inside the bladder. Coughing and straining maneuvers should then yield exactly the same recording on the chart strip, and the differential pressure channel should be zero during this maneuvers. The reference point for catheter-mounted transducers is the transducer itself. Each should be zeroed at atmospheric pressure. Although electronic calibration circuits are available for most catheter-mounted transducers, it is important to use gravity transducer calibration periodically in the daily calibration of the UPP catheter. Some investigators recommend a simple and rapid technique in which the catheter is placed in a column of water. A ruler with a centimeter scale is taped to the column of water with the zero value at the top or meniscus of the water column. As the catheter is lowered into the water, the chart strip recording response should correspond to the level of the transducer in the water column. This technique is an excellent way to ensure calibration of the microtip transducers. When calibrating the transducers with this technique, make sure that both transducers are zeroed before being placed in the water and remember that one transducer will be deeper in the water and show a higher reading than the other, depending on the distance between the transducers. Consistently low flow rates despite adequate voided volumes generally indicate increased outlet resistance, decreased bladder contractility, or both. The minimum voided volume adequate for interpretation of an accurate uroflow is generally considered 150 ml. Therefore, in an adult, flow events of less than 150 ml should be interpreted with caution. Unfortunately, a reduction in flow rate is not specific for outlet obstruction. A low flow rate does not assure bladder outlet obstruction, and a normal flow rate does not exclude it. Similarly, a maximum flow rate greater than 15 to 20 ml/sec does not absolutely exclude the possibility of outlet obstruction. In the past, measurement error caused by the uroflowmeter was occasionally a problem. Most contemporary uroflow equipment functions with reasonable accuracy and reproducibility. The uroflowmeter, however, should be tested periodically for accuracy. A simple technique involves applying a known volume of fluid at a fixed flow rate into flowmeter over a measured interval. One can calculate the mean flow by using the volume infused and the time of the infusion. This simple technique can quickly and easily identify a malfunction in the unit. One must interpret with caution data from instruments which automatically calculate maximum and average flow rates. Calculations of average flow are useful only when a clearly definable end point of micturition is apparent. In a patient with intermittent flow or significant terminal dribbling, a misleading low average flow rate may result. The urinary flow rate varies significantly with the voided volume. An important consideration therefore is to have a urinary flow rate nomogram available in the laboratory for comparison of measured flow rate and voided volume. Also, because urinary flow rates can vary in an individual from one voiding episode to another, more than one non-invasive flow rate study should be done whenever possible. Bladder over-distension may be responsible for artifacts during uroflowmetry. Temporary impairment of detrusor contractility may be caused by overstretching of the detrusor muscle fiber. A second pitfall during uroflow evaluation is that of measuring only one flow parameter, such as peak flow. This may be misleading, because some patients may generate very high peak flows with straining maneuvers. Thirdly, in comparing flow rates in a given individual from one time to another, either for the purpose of evaluating treatment or following a given condition, it is important to standardize the rates to a given volume. In cases where there is an increase in detrusor pressure accompanying bladder filling during cystometry, distinction between low bladder compliance and involuntary contractions may be difficult. Further, both conditions may exist in the same patient. A practical method for distinguishing low bladder compliance from detrusor overactivity is to stop the water inflow in the midst of observed detrusor pressure increase. If detrusor pressure drops and then stabilizes upon suspension of water inflow, decreased bladder compliance is diagnosed. If detrusor pressure continues to rise despite cessation of inflow, detrusor contraction is more likely. A common cause of an erroneous diagnosis of detrusor areflexia is failure to fill the bladder to capacity. A typical example is a patient who present with urinary retention fails multiple voiding trails and is referred for evaluation. Since most filling media comes in pre-packaged containers containing 600 or 1,000 mL, there is a tendency amongst many clinicians to discontinue bladder filling at those volumes. In our judgment, bladder filling should continue until the patient feels a strong urge to void or an uncomfortable fullness before concluding that the bladder does not contract. Failure to do so may lead to a faulty diagnosis of detrusor areflexia. Electromyography (EMG) artifacts are so common in adults that EMG signals should be interpreted with great caution and only when there is good correlation between changes in EMG and changes in detrusor pressure and uroflow. Needle EMG is much more accurate and sensitive than patch electrodes, but they are rarely used because of patient discomfort and pain. Patch electrodes should be applied to a clean, dry skin surface in the perineum and it should be assured that proper grounding has been established. Since there are no absolute units of measurement that are important, the gain should be adjusted so that the EMG tracing can be visualized properly. A weak EMG signal may result from electrodes being placed too far from the sphincter or if the gain is too low. Loss of EMG signal occurs when electrodes become wet. EMG signal will go off scale when one or more electrodes fall off the patient. In order to rule out a major malfunction of the EMG unit, a simulation test is performed using a patch placed upon the forearm of the patient or technician. Using a scale of about (±) 400, deflection of half-scale should be obtainable by slight movement of the forearm. Common EMG artifacts are: The video component of video-urodynamic studies adds anatomic perspective, but it is subject to many pitfalls. First and foremost, it is essential that the radiographic picture is correlated exactly with urodynamic tracing, and the urodynamic tracing be annotated to describe what the patient was trying to do. Pitfall relates to obtaining the proper view of the urethra. When the urethra is viewed in the anterior-posterior (AP) position, its anatomy is obscured, but when obliqued sufficiently, the shape of the urethra becomes obvious. If the X-rays are obtained in the sitting position, it is often not possible to oblique the patient sufficiently because of the excursion of the C-arm is limited by the patient's knees. Further, it is possible (and usual) that the X-ray beam is not perpendicular to the long axis of the patient. This results in the bladder appearing much lower than it really is. The reason for this is that it is often difficult to visualize the urethra unless the beam is tilted upward. Several problem areas are unresolved in urodynamics: One of most common problems encountered during pressure / flow studies is related to the urethral catheter. Intravesical pressure usually is measured with a small catheter placed through the urethra into the bladder. If the urethral pressure measurement catheter is too large, a reduced urinary flow rate and increased intravesical pressure can result, whereas a small catheter usually has little effect on the flow rate. If a urethral catheter is used for pressure measurement, the flow rate should be compared with the initial or subsequent uroflow tracing obtained without a catheter to determine if the pressure catheter has a significant effect. Another problem with pressure / flow studies is that the urethral pressure catheter may be expelled during voiding. The urethral catheter can also deflect the stream away from the flowmeter. It is important to remember that urodynamic studies are very interactive and dynamic. They should be performed to address a specific question or questions, and should attempt to reproduce the patient's symptoms. It is important to remember that urodynamic studies represent a snapshot at a single point in time, and as such may not be representative of the patient's symptoms or pathophysiology. The patient should be asked whether or not the urodynamic study was representative of his usual symptoms and the data interpreted with this in mind. A good urodynamic study will reproduce the patient's symptoms; a good quality tracing with appropriate annotations will allow for a reproducible interpretation. |