إصابة المسالك البولية: الوقاية والإدارة
[وهك] ممارسة نشرة وسريرية إدارة لمقدمي الرعاية الصحية. قدمت منحة للتربية وصحة المرأة وتربية مركز ([وهك]).
The evolution of pelvic surgical procedures have been influenced by uncommon but potentially devastating injuries to the lower urinary tract (bladder and ureters). Most gynecological procedures carry an identifiable risk of visceral organ injury. These injuries are associated with known risk factors, though not all such injuries are predicable or avoidable. Accurate diagnosis and safe, timely repair of bowel, bladder and ureter is crucial in reducing morbidity, and potential mortality. Timely and effective repair of these injuries is critical in improving patient outcomes and mitigating litigation risk, as claims relating to intraoperative injury to pelvic organs have high average indemnity payments and more frequently end in payments than do other gynecologic complications. It is essential that gynecologic surgeons have a high suspicion for visceral injury, are prepared to diagnose these injuries intraoperatively, can safely repair injuries with or without consultants, and can follow patients postoperatively in a careful manner. Despite best efforts, occasional injury to the urinary tract during obstetrical and gynecologic surgery is inevitable. It is important to remain vigilant and aware of the possibility of injury at the time of surgery to better optimize patient outcomes.
The purpose of this review is to focus on the literature regarding the incidence, diagnosis and management of injuries to the lower urinary tract. Diagnosis of such injuries requires careful attention to surgical entry and dissection techniques and employment of adjuvant diagnostic modalities. The participation of consultants is encouraged depending on the primary surgeon's skill and expertise. Postoperative care after bladder and ureteral injury requires surveillance for complications including repair site leak, abscess and fistula formation.
Incidence
Overall the incidence of lower urinary tract injuries has not been established, but it is estimated that benign gynecologic surgery, bladder injury rates range from 0.3 to 6.0 per 1,000 hysterectomies with or without bilateral salpingo-oophorectomy; ureteral injuries range from 0.2 to 7.3 per 1,000 hysterectomies. Obstetric and gynecologic procedures account for 65% of all non-endoscopic iatrogenic bladder injuries across surgical specialties. Urinary tract injuries occur during 0.33% of all benign gynecologic laparoscopies and 0.73%-1.8% of laparoscopic hysterectomies (1). Bladder injuries occur during 0.24% of gynecologic laparoscopies, 1.3% of vaginal hysterectomies. Composite bladder injury rates during hysterectomies have been reported as high as 3.6% (2).
While bladder injury occurs in uncomplicated cases, injuries can be associated with several patient-related and perioperative factors such as the performance of concurrent anti-incontinence procedures or lysis of adhesions (3). The presence of endometriosis has been suggested as a risk factor for bladder injury during laparoscopic hysterectomy. As cesarean delivery rates and surgical correction of urinary incontinence and pelvic organ prolapse continue to rise, significant numbers of lower urinary tract injuries are occurring each year.
Anatomy
Knowledge of anatomy and careful intraoperative ureteral identification are hallmarks in injury prevention. The pelvic ureter is easily identified from the pelvic brim to uterine artery. The length of the adult female ureter ranges from 22 to 28 cm. After crossing under the uterine artery, it enters and parametrium and courses within a "ureteral tunnel," where it roughly separates the anterior fibers of the uterosacral ligament. In this region, the ureter lies along the anterolateral cervix and upper vaginal wall to enter the bladder. Lastly, it courses within the bladder wall to terminate at the ureteric orifice. See Figure 1 below.
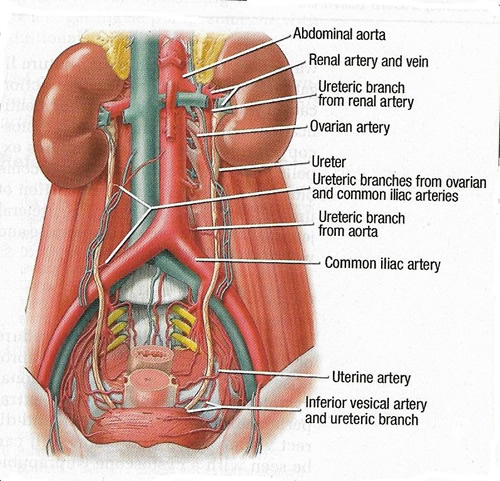
Figure 1. The course of the ureter as it descends into the pelvis.
Data suggest that ureteral injury most commonly occurs while securing the uterine artery and with the division of the cardinal ligament during hysterectomy (4). Understanding the ureter's anatomy within the parametrium and its relationship to other pelvic structures is critical to safely complete gynecologic surgery.
This study (5) found the ureter to be an average of 0.8 cm from the ovarian vessels at the pelvic brim, 1.7 cm from the from the uterine isthmus, and 1.3 cm from the lateral vaginal apex; these findings support previous observations that the ureter is at greater risk of injury during the following steps of a hysterectomy: ligation of the ovarian vessels, transection of the uterine artery and cardinal ligament, and suturing of the vaginal cuff. It is suggested a substantial injury risk is added when clamps are placed beyond 1.5 cm lateral to the uterine isthmus or 1 cm lateral to the vaginal cuff.
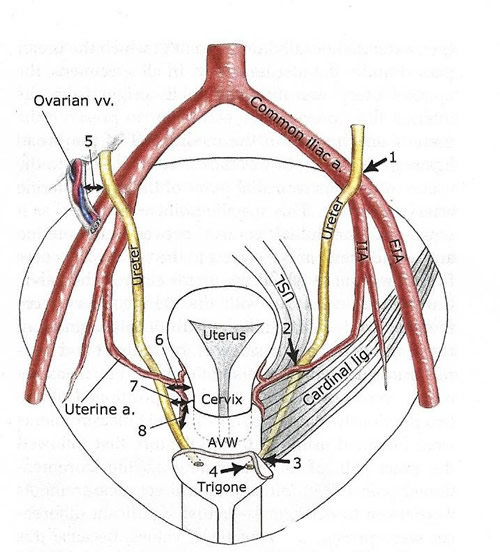
1 - lateral border of ureter as it crosses over iliac vessels:
2 - superomedial point where ureter passes under uterine artery;
3 - point at which ureter enters bladder wall;
4 - ureteric orifice;
5 - lateral ureter to most medial ovarian vessel at pelvic brim;
6 - medial ureter to uterine isthmus;
7 - medial ureter to anterolateral vaginal fornix (vaginal apex in specimens without uteri);
8 - distance from ureter as it passes over anterolateral vaginal wall to anterolateral vaginal fornix-vaginal apex; Anterior vaginal wall (AVW); external iliac artery (EIA); internal iliac artery (IIA); uterosacral ligament (USL).
Ureter, a retroperitoneal structure can be divided into thirds. The proximal third arises from the renal pelvis and descends along the ventral surface of the psoas muscle. In its midportion, the ovarian vessels cross the ureter anteriorly as the ureter approaches the pelvic brim. The ureter then passes over the bifurcation of the internal and external iliac vessels. The distal third of the ureter descends into the pelvis, where it lies in a connective tissue sheath and is loosely attached to the medial leaf of the broad ligament. The ureter is least likely to be injured where it is seen traveling and its peristalsis seen in the middle leaf of broad ligament. It then passes through the cardinal ligament, traveling along the upper anterior vagina, passing within the bladder wall, and inserting to form a ureteral orifice.
Types of Urologic Injuries
Bladder: contusion; thermal injury; laceration; perforation; erosion of suture or mesh; and intravesical suture or mesh.
Ureteral: contusion; thermal injury; laceration; transection; ligation; kinking; and devascularization.
Urethral: laceration; perforation; erosion of mesh and intraurethral mesh.
Preventing Urinary Tract Injury
Hysterectomy remains the most common gynecologic procedure in the United States; approximately 600,000 hysterectomies are performed every year, the majority of which are for benign diseases (6). Increased proficiency with laparoscopic hysterectomy will lead to decreased rates of urinary tract injury. Strategies discussed in this review to minimize risk of injury apply to all operative approaches.
Strategy 1: Possess knowledge of the anatomy
The course of ureter in the deep pelvis necessitates isolation and lateralization of the ureter in cases of total or radical hysterectomy in order to avoid injury. Nearly 80% of ureteral injuries occur in close proximity to the uterine artery (7). The bladder trigone and bladder base are also at risk of injury in the deep pelvis. The trigone rests over the anterior bladder fornix and the bladder base rests on the lower uterine segment and cervix (8).
Strategy 2: Address patient-specific risk factors
In addition to knowledge of the anatomy and meticulous dissection, preoperative planning is essential to minimize risk of urinary tract injury. Half of all patients who sustain a ureteral injury have no identifiable risk factors, but if patient-specific issues are identified, additional imaging studies or alterations in the surgical plan can be considered to mitigate risk. Risk of injury to the urinary tract is higher in procedures for invasive cancer or urogynecologic surgery. 10% of patients undergoing hysterectomy for known cervical pathology (such as mass or tumor) will have ureter within 5 mm of the cervical tissue (9). In these individuals, preoperative imaging may be helpful for surgical planning in an attempt to minimize risk of ureteral injury.
Pelvic anatomy also may be distorted in association with the particular clinical scenarios. For example, pelvic adhesive disease, may be the initial barrier to visualizing and isolating pertinent anatomy in patients with a history of abdominal pelvic surgery, pelvic radiation, pelvic infections or advanced endometriosis. Multiple cesarean deliveries are also associated with an increased risk of pelvic adhesive disease (10). In such cases, sharp dissection is preferable to blunt dissection or use of thermal instruments to minimize risk of injury in the setting of compromised anatomic planes. Patients undergoing hysterectomy for a large uteri or who require resection of adnexal masses are also at increased risk of ureteral injury (11).
Ureteral catheterization (stent placement): It has been proposed in high-risk populations and has also been investigated as universal preoperative prophylaxis. In both a retrospective study and randomized trial, use of catheterization resulted in no significant difference in incidence of ureteral injury (12). In a separate retrospective study found a decreased incidence of ureteral injury with preoperative stent placement, in addition of a cost savings in terms of operative time related to identification of the ureter (13). Based on these findings, we suggest that prophylactic ureteral catheterization should not be a substitute for meticulous dissection, but in an appropriately selected patient it may improve the ability to identify the ureter either visually or by palpation. The decision about use of ureteral catheterization should be left to the surgeon's discretion.
Strategy 3: Screen for injury
Minimizing the risk of intraoperative injury requires maintaining visual identification of the ureters and bladder in relation to the operative target. This also allows early recognition of injury, should it occur. Confirmatory measures for further reorientation include palpation of the ureters and bladder, bladder back-filling, administration of intravenous (IV) dye, cystoscopy and retrograde pyelography. Sluggish or absent efflux of urine from the ureteral orifices is often a sign of ureteral injury. Ureteral efflux is also not a guarantee of ureteral integrity in cases of partial obstruction. Although cystoscopy may be a useful adjunct to aid in detection of bladder or ureteral injury, it should not be considered a substitute for proper surgical technique and intraoperative visualization and isolation of these structures.
After sling and other retropubic procedures, cystourethroscopy should be performed to assure the absence of mesh material in the bladder or urethra. The most typical location for injury is in the dome of the bladder, which is why it is particularly helpful to visualize the path of the trocar needles along the lateral aspect of the dome. This can be done by manipulating the needle and observing its course with a 70° cystoscope. Injury to the trigone is uncommon with midurethral slings, particularly tension-free vaginal tape. Rarely, there may be an injury to the urethra, which is best evaluated with a 0° cystoscope.
Strategy 4: Recognize the injury
Delayed identification of urinary tract injury can result in poor outcomes with long-standing sequelae such as compromised or lost renal function. Intraoperative or postoperative consultation with a urologist is recommended and may be necessary in complex cases, even when the gynecologic surgeon is capable of ureteric or bladder repair. The approach of immediate repair is dependent on the type of injury, with crush or thermal injuries requiring resection of the damaged segment. In cases of delayed diagnosis, placement of a nephrostomy tube may be required as a temporizing measure prior to definitive repair.
Postoperatively, a high degree of suspicion is required to identify patients with urinary tract injuries unrecognized at the time of surgery. Patients may present with a wide range of complaints, depending on the time since the primary surgery. Symptoms may include flank pain or costovertebral angle tenderness, fever, ileus, peritonitis, anuria, or frank fistula (14),(15). Computed tomography (CT) imaging aids in postoperative diagnosis of urinary tract injury by its ability to detect intra-abdominal extravasation of urine (15). Fluoroscopic retrograde ureterography and urogram with IV contrast are additional methods of identifying urinary tract patency postoperatively (16).
Symptoms of Urologic Injuries
Bladder: Profuse drain output, profuse wound leakage, ileus, fever, peritonitis and hematuria.
Ureteral obstruction: Flank or abdominal pain, anuria.
Fistula formation: Urinary incontinence, watery vaginal discharge.
Intraureteral or intravesical mesh: Hematuria, dysuria, recurrent urinary tract infection, De novo urinary urgency, urge incontinence and pelvic pain.
Management
Bladder Injuries
Cystotomies are a known complication of gynecologic procedures that involve mobilizing the bladder flap off the uterus, cervix, and vagina. In this study the highest rates of injuries were to the bladder, ranging from 0.05% to 0.66%. Total laparoscopic hysterectomy had the highest injury rate, and supracervical hysterectomy the lowest. The majority of cystotomies (80.6%) were recognized intraoperatively, whereas 7.5% were recognized postoperatively. The conversion rate to laparotomy to repair bladder injuries was 11% (17). Signs of a cystotomy during surgery may include an air-filled Foley catheter drainage bag that is usually identified by the anesthesia team as the result of the abdominal insufflation passing through the cystotomy to the bag, which is at atmospheric pressure. Other signs are blood-tinged urine in the Foley bag and difficulty visualizing the bladder walls when cystoscopy is performed. Blood clots in the bladder should be irrigated gently and removed. A clot at the base of the bladder may occlude the bladder defect and prevent fluid loss from the bladder.
Bladder integrity can be assessed by distending the bladder with methylene blue, sterile milk, or saline. Carbon dioxide can similarly be instilled using the insufflation tubing that establishes pneumoperitoneum (18). Distension of the bladder should be performed under direct laparoscopic guidance, generally with instillation of 200-250 mL liquid or gas. This approach is most appropriate when cystoscopy is not anticipated. Alternatively, cystoscopy may be used to evaluate bladder when an assessment of ureteral patency is already planned. This approach allows for localization of an injury and assesses proximity to the trigone. The bladder and ureteral orifices are best evaluated with a 70° or 30° scope.
It is appropriate to seek urologic consultation when there has been an injury to the trigone of the bladder. Proximity to the ureters is important when considering repair of the bladder trigone, and ureteral reimplantation may be required. Furthermore, consideration of the final position of the ureteral orifice is typically out of the scope of a general gynecologic surgeon.
Closing a bladder cystotomy: Once a cystotomy is identified by cystoscopy, the treating surgeon can visualize it intra-abdominally by elevating the posterior bladder wall and looking for the fluid leak, aided by the light from the cystoscope. Visualization of the bladder edge is facilitated when the bladder is full. For this reason, placing a 3-way Foley catheter (at least 22F) that is connected to a 5-L fluid source and clamping the output channel can aid in laparoscopic closure of the cystotomy.
Closure of the cystotomy can be performed using an absorbable barbed suture on both layers, eliminating knot tying (19). The first layer is closed with care taken to incorporate the lateral edges of the incision, where a small piece of bladder mucosa may be inadvertently puckered outside of the suture line. Angling the sutures at 45° at the lateral edges can prevent this complication. Take care to ensure that each bite is through bladder mucosa. Typically, a larger bite of mucosa and a smaller bite of the bladder muscularis are sufficient for the first layer.
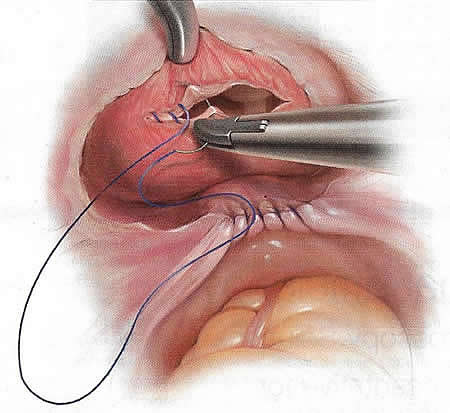
Figure 3. Double-layered closure of a cystotomy using absorbable barbed suture, with care taken to incorporate the lateral edges of the incision.
Once the first layer is closed, the bladder should be sufficiently emptied to allow for placement of the second row of sutures tension-free. The purpose of the second layer is to imbricate the first layer completely. Therefore, the starting and ending edges of the second layer must be lateral to the first layer. The second suture line should include the bladder serosa and the bladder muscularis. Once 2 layers are closed, inspect the suture line with the bladder full. A repeat cystoscopy is not necessary unless there is a concern for a bladder clot. Gentle irrigation of a clot will dislodge it.
Completing the sacrocolpopexy, or not: Whether or not to proceed with the sacrocolpopexy is a decision the operating surgeon makes. Because it is essential that the cystotomy suture line be tension-free, prior to starting the cystotomy repair some surgeons place a Lucite stent in the vagina to aid dissection and mobilize the bladder, making certain that the cystotomy is 2 to 3 cm proximal to the distal edge of the dissection. This is important because tensioning of the sacrocolpopexy mesh could inadvertently place tension on the cystotomy repair. A third layer of closure may be indicated if an omental or peritoneal flap cannot be placed between the bladder closure and the anterior vaginal wall mesh.
Midurethral sling placement, or not: Surgeon preference dictates whether or not to proceed with a midurethral sling. Most of the surgeons do not place a sling whenever there is cystotomy at the posterior bladder wall or bladder dome. Once the catheter is removed, the bladder wall may be subjected to increased pressure due to increased urethral resistance if a sling has been placed. In some cases, higher than normal bladder pressures or frank obstruction may increase the risk that the cystotomy repair will break down. Given the low morbidity associated with performing the sling procedure at a later date; most of the surgeons defer sling placement until 2 to 3 months following the cystotomy repair.
How long to leave the Foley catheter: A Foley catheter is typically left in place for 1 to 2 weeks. Keeping the bladder nondistended minimizes the likelihood that the posterior bladder wall will drape over the mesh. Most of the surgeons do not perform a cystogram on removal of the Foley catheter, although some authors advocate this (20).
After bladder repair, patients may develop discomfort or bladder spasm due to the temporary presence of suture and indwelling Foley catheter. This can be managed with anticholinergic agents such as oxybutynin or belladonna-opium suppositories.
Ureteral Injuries
Ureteral injury can occur in several ways: thermal or crush injury, suture ligation, transection, or adjacent traction causing kinking. The mainstay for identification of ureteral injury includes cystoscopy. Of ureteral injury cases described in the literature, transection was the most common, and the majority occurred at the pelvic brim (21).
Intravenous indigo carmine is expelled in the urine, causing it to become blue, which aids in the visualization of ureteral jets. Failure to visualize ureteral jets may suggest ureteral compromise and warrants further investigations. Agents used to improve detection of the ureteral jets during cystoscopy are:
- Dextrose 10% solution as cystoscopy fluid. The fluid viscosity difference makes ureteral jets easier to detect.
- Indigotindisulfonate sodium (Indigo Carmine) 0.8% solution, 5 mL ampule given intravenous (IV) 10 minutes prior to cystoscopy. Has a half-life of 5 minutes.
- Indocyanine green (ICG: IC-Green) 25 mg/10 mL (off-label use) given IV 2-3 minutes prior to cystoscopy (22).
- Phenazopyridine (Pyridium) 200 mg orally with sip of water in preoperative holding area.
Intraoperative versus Postoperative Detection
The rate of ureteral injury is higher with intraoperative versus postoperative detection. The rate of intraoperative detection was 0.6% with use of cystoscopy in 3,235 patients and 6 times lower (0.1%) for postoperative detection in 107,068 in this study (23). In two other studies, 89% and 93.7% of ureteral injuries, respectively, were not detected intraoperatively (24). Intraoperative cystoscopy identified about 90% of unrecognized ureteral injuries, 69% of which were easily managed, most by simply removing a suture (23).
One reason for the lower rate of detection of postoperative ureteral entrapment is the lack of symptomatology following ureteral ligation. Actually, there are no methods other than ureteral dissection and identification to prevent any type of ureteral injury. Ureteral entrapment may be asymptomatic, or patients may present within 1 week with flank pain or fever due to pyelonephritis. Thermal injuries may be diagnosed as long as 2 to 3 weeks after surgery.
Delayed Postoperative Recognition: Unilateral ureteral obstruction and fistula formation will usually present later in the postoperative course. Ureteral obstruction may cause abdominal or flank pain or may be asymptomatic, resulting in later diagnosis of a non-functioning kidney. Fistula formation typically results in urinary incontinence, watery vaginal discharge, or both.
Mechanisms of Injury: Entrapment results in increased renal pelvis pressure within 4 hours, distal tubal atrophy in 1-week, proximal tubular atrophy in 2 weeks, and progressive glomerulo-sclerosis over 4 weeks, and permanent damage unless corrected within this period of time (25). Transection and thermal damage result in urine extravasation (urinoma) and chemical peritonitis. Prior to endoscopic surgery, thermal ureteral injuries were almost non-existent, and they became quite common with introduction of electrical instrumentation, whether monopolar or bipolar. In the latter case, it is not the electrical current, but the steam generated from the application of the electrical current, with secondary boiling of the intracellular and extracellular fluids, that results in thermal injury.
Diagnosis: During surgery, different methods are used to inspect the ureter and bladder. Some surgeons only perform gross examination of the lower urinary tract. Ureteric peristalsis alone does not equal ureteric patency, as a damaged ureter will continue to demonstrate peristalsis. According to one prospective study, peristalsis persisted in 5 out of 6 ureteric injuries (26). Additional methods to evaluate the lower urinary tract are available: for example, bladder instillation of methylene blue dye, IV indigo carmine, ureteric stents, and/or cystoscopy.
Urography (or pyelography) provides information on the structure and functionality of the urinary system and is particularly helpful in identifying ureteral stricture or obstruction. The absence of contrast media within the renal pelvis at the time of intravenous pyelography is concerning for a non-functioning kidney. Intravenous pyelography may be combined with CT or magnetic resonance imaging (MRI) for additional information and is now considered the standard for evaluation of stricture or obstruction. Retrograde pyelography is performed in conjunction with cystoscopy, because contrast media is injected directly into the ureter to assess for stricture or obstruction. A combination of CT intravenous pyelography and retrograde pyelography may be necessary to appreciate the full extent of an obstruction or stricture.
Ureteral injury may be suspected based on renal, pelvic, and bladder ultrasonography and then localized with CT intravenous pyelography (23). Retrograde pyelography is most helpful when CT intravenous pyelography results are inconclusive (28).
Repair of Ureteral Injuries
When there is obstruction to the flow of urine from the kidney, the first priority is to either restore or divert flow to avoid long-term renal damage. This is particularly important when a pelvic infection or abscess is present, because repair will need to be delayed until the infection has been treated. In the absence of infection, reoperation may be undertaken immediately after diagnosis, when operative management is deemed appropriate.
Kinking can often be resolved by simple removal of a suture and requires no further management. Ligation and crush injuries can result in ureteric damage and devascularization. Ureteral stent may be sufficient for minor damage, whereas resection may be required for more extensive damage. Crush injuries can result in significant amount of tissue damage depending on the size, type, and duration of clamp placement. The decision to stent or resect should take those variables into account.
Lacerations can be repaired with interrupted stitches, repaired as a complete transection with reanastomosis, or reimplanted into the bladder. A laceration less than half the diameter of the ureter can be repaired over a stent with interrupted delayed absorbable suture. Injury to more than half the diameter of the ureter requires either anastomosis or reimplantation. Stent placement is used in all of these scenarios to promote ureteral healing, prevent urine extravasation, and to avoid structuring. A ureteric stent should therefore be placed when reimplanting the ureter or reestablishing continuity (28). A drain should also be placed in proximity to the anastomotic site to inform the health care provider of leakage.
When planning repair of a complete transection, the location of the injury needs to be considered. The ureter is divided into proximal, middle, and distal thirds. The proximal portion of the ureter extends from the ureteropelvic junction to the upper border of the sacroiliac joint, whereas the middle portion extends from the upper border to the lower border of the sacroiliac joint, and the distal portion extends from the inferior border of the sacroiliac joint to the ureterovesical junction. Injuries to the distal third of the ureter are typically managed by reimplantation, especially when in close proximation to the bladder where a ureteral anastomosis would be technically challenging.
Thermal injury or ischemia to the ureter from adventitial dissection can result in scar tissue formation, stricture and obstruction. Minor thermal injuries may be managed by ureteral stent placement. Extensive injury should be managed with resection and reparative surgery commensurate with the location of the injury. A flap of omentum can be interposed between bowel and ureter repair site, if it is in close proximation, to avoid fistula formation or anastomosis breakdown, and it is particularly important when thermal and ischemic injuries occur (29).
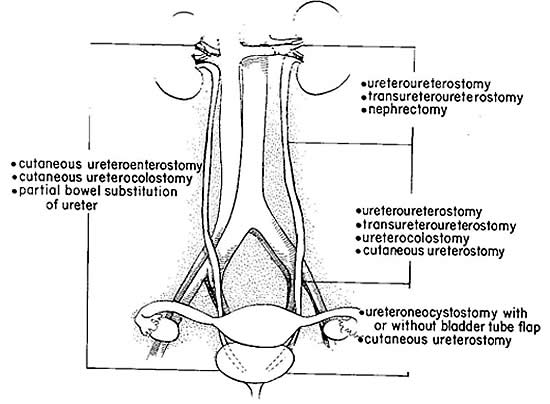
Figure 4. Surgical procedures recommended for repair of ureteral injuries, according to involved ureteral segment.
Postoperative care and follow up: Maintaining adequate drainage of the urinary system with a Foley catheter and a ureteral stent is critically important to the healing of ureteral injuries. When the ureter is reimplanted, a Foley catheter should be used to maintain bladder drainage. It may be removed 1-2 weeks postoperatively on cystogram confirmation of absence of leakage (30). Ureteric stents after anastomosis or reimplantation may be removed 1-2 months postoperatively. Stent removal should be directly followed by either cystogram or intravenous pyelography to assess the anastomotic site (30). At 3-6 months, and again at 12 months, the anastomotic site should be assessed for stricture and appropriate kidney function confirmed (30). This can be done with a combination of intravenous pyelography, renal ultrasonography, and serum creatinine.
Secondary leaks after a primary repair should be managed with percutaneous nephrostomy tube drainage and antegrade ureteral stents when possible, because retrograde stenting is often unsuccessful. Urinomas can be managed with percutaneous drain placement. Ureteral stents are not without complications and even correctly placed stents can be accompanied by irritative voiding symptoms, flank pain, suprapubic discomfort, hematuria. More severe complications can result for stenting-induced trauma and include ureteral and parenchymal perforation with associated hemorrhage. Some injuries discovered postoperatively can be managed through drainage techniques, whereas others will require a return to the operating room.
Urethral and Periurethral Injuries
The female urethra, on average, ranges in length from 3 to 5 cm and is approximately 6 mm in diameter. The posterior urethra is intimately attached to the anterior vaginal wall. It is primarily lined with stratified squamous epithelium and is surrounded by smooth and striated muscle (31). In obstetrics, urethral and periurethral injuries usually occur at the time of vaginal delivery, while in gynecologic surgery it most commonly occurs during midurethral sling placement. In both cases, injuries can be identified by direct visualization; however, cystourethroscopic evaluation may aid in determination of the extent of the injury. Visualization and evaluation of the urethra can best be accomplished using a cystoscope with a 0° lens.
Ten years after introduction of midurethral slings for treatment of stress incontinence more than 1.2 million midurethral slings had been implanted worldwide (32). Although complication rates reported in clinical trials are very low, in the range of 1% to 6 %, the release of the U.S. Food and Drug Administration's (FDA) advisory on use of pelvic mesh in 2008 and Health Canada's warning in 2010, resulted in increased attention from patients and media regarding suboptimal outcomes after these procedures (33).
Minimizing Risks: Risk of urethral injury can be minimized with controlled delivery of the fetus and appropriate downward traction during operative vaginal delivery. Suburethral injection (hydrodissection) at the time of midurethral sling placement may also help in avoiding urethrotomy by increasing the distance from the vaginal mucosa to the urethra. Foley catheter placement can also aid in identification of the posterior urethral wall during palpation and incision. Lastly, use a rigid catheter guide during midurethral sling placement helps protect the urethra and bladder neck by deflecting them during trocar passage.
The rates of revision surgery after insertion of mesh midurethral slings and explore whether physician specialty, annual operative volume, or hospital type are associated with good outcomes. This study concluded, 1 in 20 women underwent revision surgery within 10 years after midurethral sling placement. Higher physician surgical volume is associated with decreased risk, with the decline occurring at a threshold of 50 cases annually (34). Minimum caseload parameters for surgeons performing midurethral sling procedures may improve quality of these procedures.
Management
Small, superficial urethral and periurethral lacerations can be reapproximated with 4-0 absorbable suture. Placement of Foley catheter prior to repair can aid in avoiding unintentional narrowing of the urethral meatus. Depending on the extent of urethral injury and the surgeon's comfort level with urethral repair, it may be necessary to obtain the help of a specialist in these cases.
In the case of urethral injury at the time of midurethral sling placement, the urethra can be repaired in a 2-layer fashion using and initial layer of 4-0 or 5-0 absorbable suture. It may be necessary to mobilize some periurethral tissue for the second layer, which can be a 3-0 or 4-0 absorbable suture. Depending on the location of the injury, it may be advisable to avoid placement of the sling at this time, as the injury combined with concurrent sling placement may increase the risk of mesh erosion into the urethra or fistula formation. Catheter placement for 10 to 14 days after urethral reconstruction is recommended to avoid urethral stricture.
UROGENITAL FISTULAE
Of all the non-fatal complications of gynecological surgery, fistula is the one that gynecologists the world over seem to fear most, although ureteric injury runs a close second (35). Fistulae can form among a variety of pelvic structures and can result from many different causes. In developed countries, the incidence of fistula formation after hysterectomy has been estimated at 0.1% with vesicovaginal fistulae occurring in 1 out of 455 to 1,800 hysterectomies (35). In this population study of nearly 300,000 women undergoing a hysterectomy for benign indications, a combined genitourinary injury incidence was 18 per 1,000 patients (1.8%) (36). This study also explored the effect of timing on repair. Observation was most injuries (76.4%) were identified at the time of surgery and immediately repaired. Immediate identification and repair is associated with a reduced risk of subsequent genitourinary fistula formation (36).
The various urogenital fistulae are discussed in separate sections.
Surgical Management of Lower Urinary Tract Fistulae
http://www.womenshealthsection.com/content/urogvvf/urogvvf007.php3
Managing Vesico-Vaginal Fistulae
http://www.womenshealthsection.com/content/urogvvf/urogvvf002.php3
Recto-Vaginal Fistulae and Fecal Incontinence
http://www.womenshealthsection.com/content/urogvvf/urogvvf008.php3
Summary:
Urinary tract injury is a known complication of hysterectomy, regardless of route of procedure. Surgeon familiarity and comfort with complex anatomy, as well as preoperative risk stratification, is essential to minimizing risk of urinary tract injury. Intraoperative assessment of ureter and bladder integrity is the first step in preventing delayed diagnosis of injury. Although a useful adjunct, cystoscopy does not identify all injuries. A high index of suspicion postoperatively with appropriate imaging will promote early diagnosis of intraoperative injuries. Consultation with an advanced gynecologic surgeon and/or urologist is recommended when definitive repair of injury may require additional interventions prior to corrective surgery. Identifying the injury intraoperatively reduces postoperative complications and long-term sequalae. The use of cystoscopy and agents that allow for easy discernment of ureteral efflux aid in identifying urinary tract injuries intraoperatively.
References:
- Wong JMK, Bortoletto P, Tolentino J, et al. Urinary tract injury in gynecologic laparoscopy for benign indication: a systematic review. Obstet Gynecol 2018;131:100-108
- Satiniramai D, Manonai J. Urologic injuries during gynecologic surgery, a 10-year review. J Obstet Gynecol Res 2017;43:557-563
- Adelman MR, Barsley TR, Sharp HT. Urinary tract injuries in laparoscopic hysterectomy: a systematic review. J Minim Invasive Gynecol 2014;21:558-566
- Sharp HT, Adelman MR. Prevention, recognition, and management of urologic injuries during gynecologic surgery. Obstet Gynecol 2016;127:1085-1096
- Jackson LA, Ramirez DM, Carrick KS, et al. Gross and histologic anatomy of the pelvic ureter. Obstet Gynecol 2019;133:896-904
- Wu JM, Wechter ME, Geller EJ et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110(5):1091-1095
- Liapis A, Bakas P, Giannopoulous V, et al. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(6):391-393
- Rock JA jr, Jones HW. TeLinde's Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008
- Gemer O, Simonovsky A, Huerta M, et al. A radiological study on the anatomical proximity of the ureters and the cervix. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(9):991-995
- Tulandi T, Agdi M, Zarel A, et al. Adhesion development and morbidity after repeat cesarean delivery. Am J Obstet Gynecol 2009;201(1):56.e1-56e6
- Dandolu V, Santos L, Ohana E, et al. Accuracy of cystoscopy in the diagnosis of ureteral injury in benign gynecologic surgery. Int Urogynecol J Pelvic Floor Dysfunct 2003;14(6):427-431
- Chou MT, Wang CJ, Lien RC. Prophylactic ureteral catheterization in gynecologic surgery: a 12-year randomized trial in a community hospital. Int Urogynecol J Pelvic Floor Dysfunct 2009;20(6):689-693
- Redan JA, McCarus SD. Protect the ureters. JSLS 2009;13(2):139-141
- Kim JH, Moore C, Jones JS, et al. Management of ureteral injuries associated with vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2006;17(5):531-535
- Gayer G, Hertz M, Zissin R. Ureteral injuries: CT diagnosis. Semin Ultrasound CT MR 2004;25(3):277-285
- Wu HH, Yang PY, Yeh GP, et al. The detection of ureteral injuries after hysterectomy. J Minim Invasive Gynecol 2006;13(5):403-408
- Adelman MR, Bardsley TR, Sharp HT. Urinary tract injuries in laparoscopic hysterectomy: a systematic review. J Minim Invasive Gynecol 2014;21:558-566
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol 2009;16:195-197
- Chamsy D, King C, Lee T. The use of barbed suture for bladder and bowel repair. J Minim Invasive Gynecol 2015;22:648-652
- Inaba K, Okoye OT, Browder T, et al. Prospective evaluation of the utility of routine post-operative cystogram after traumatic bladder injury. J Trauma Acute Care Surg 2013;75:1019-1023
- Ostrzenski A, Radolinski B, Ostrzenska KM. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv 2003;58(12):794-799
- Doyle PJ, Lipetskaia L, Duecy E, et al. Sodium fluorescein use during intraoperative cystoscopy. Obstet Gynecol 2015;125(3):548-550
- Janssen PF, Brolmann HA, Huime JA. Causes and prevention of laparoscopic ureter injuries: an analysis of 31 cases during laparoscopic hysterectomy in the Netherlands. Surg Endosc 2013;27(3):946-956
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol 2015;22:1278-1286
- El-Tabey AN, Ali-E-Dein B, Shaaban AA, et al. Urological trauma after gynecological and obstetric surgeries. Scand J Urol Nephrol 2006;40:225-231
- Lim MC, Lee BY, Lee DO, et al. Lower urinary tract injuries diagnosed after hysterectomy: seven-year experience at a cancer hospital. J Obstet Gynecol Res 2010;36(2):318-325
- Brandes S, Coburn M, Armenakas N, et al. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int 2004;94:277-289
- Stein R, Rubenwolf P, Ziesel C, et al. Psoas hitch and Boari flap ureteroneocystostomy. BJU Int 2013;112:137-155
- Pomeo A, Molina WR, Sehrt D, et al. Laparoscopic ureteroneocystostomy for ureteral injuries after hysterectomy. JSLS 2013;17:121-125
- Choi KM, Choi JS, Lee JH, et al. Laparoscopic ureteroureteral anastomosis for distal ureteral injuries during gynecologic laparoscopic surgery. J Minim Invasive Gynecol 2010;17:468-472
- Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. Philadelphia, PA: W.B. Saunders, 2006
- Ross S, Tang S, Eliasziw M, et al. Transobturator tape versus retropubic tension free vaginal tape for stress urinary incontinence: 5-year safety and effectiveness outcomes following a randomized trial. Int Urogynecol J 2016;27:879-886
- Food and Drug Administration. FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. Available at: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants Accessed on 20 June 2019
- Brennand EA, Quan H. Evaluation of the effect of surgeon's operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol 2019;133:1099-1108
- Rogers RG, Jeppson PC. Current diagnosis and management of pelvic fistulae in women. Obstet Gynecol 2016;128:635-650
- Dallas KB, Rogo-Gupta L, Elliott CS. Urologic injury and fistula formation after hysterectomy for benign indications. Obstet Gynecol 2019;134:241-249
نشر: 6 August 2019
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


